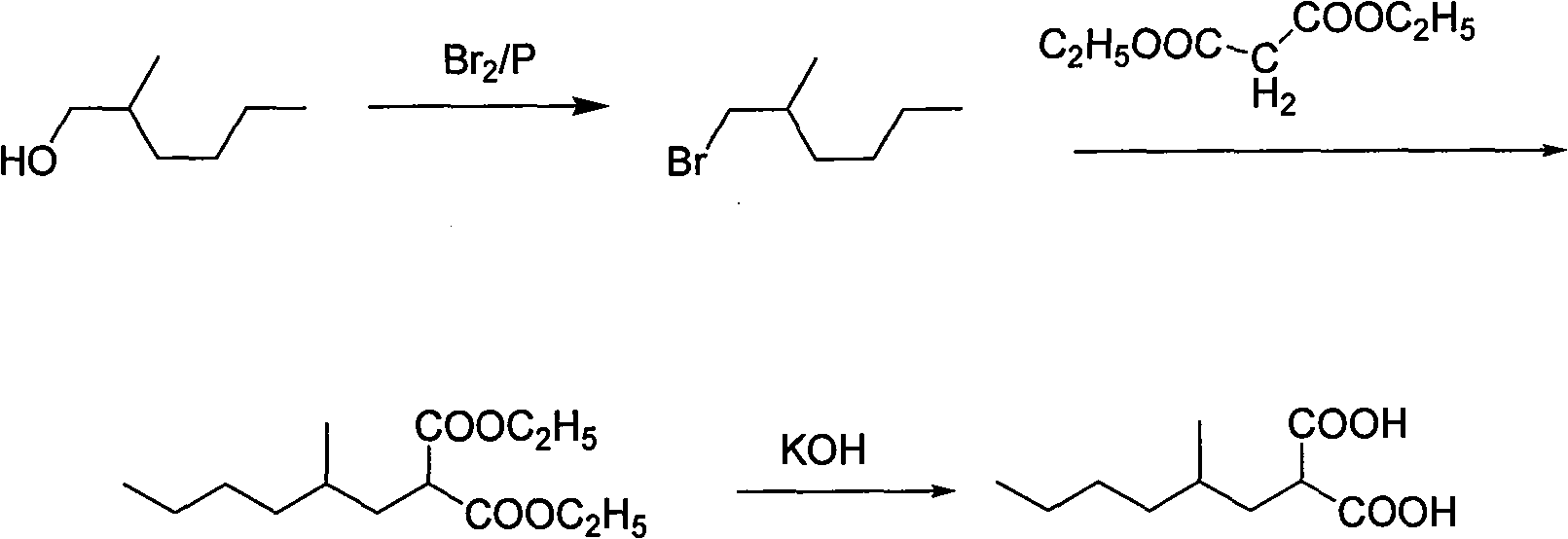
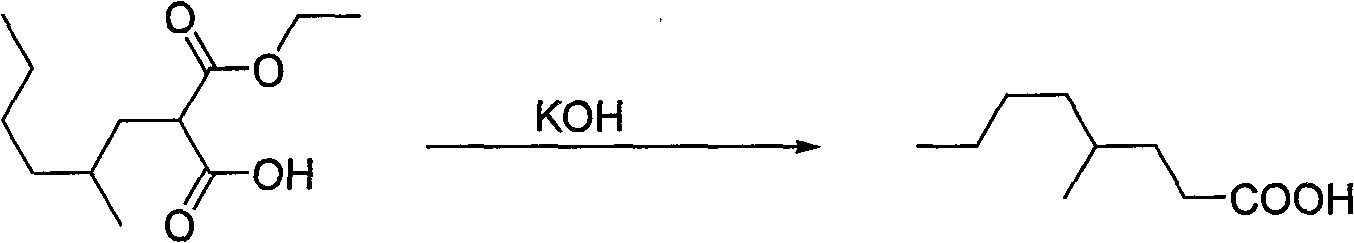
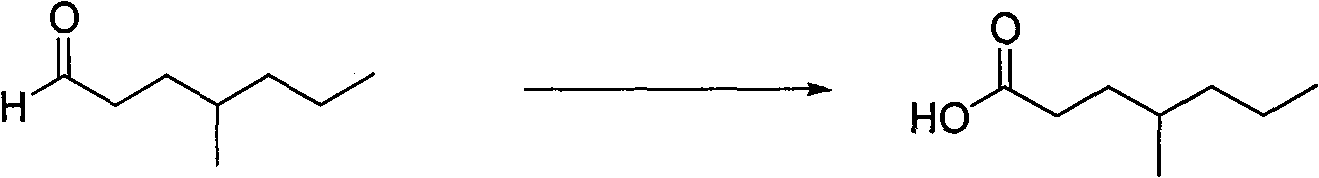Process for preparing 4-methyl caprylic acid
A technology of methyl caprylic acid and methyl group, which is applied in the field of synthesis of spice 4-methyl caprylic acid, and achieves the effects of mild reaction conditions, high total yield and convenient post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) At 0°C, after mixing 20.6 g (0.35 mol, 24.24 mL) allyl alcohol and 200 mL n-pentane, add dropwise to 1.6M n-butyllithium (0.80 mol, 500 mL) within 20 minutes, and stir at room temperature React for 1 hour. At 0°C, carefully add 200 mL of water to the reaction system, stir the reaction for 20 minutes, separate the layers, wash the organic layer with 30 mL of 3M hydrochloric acid, and then wash twice with 30 mL of water, dry the organic layer with anhydrous magnesium sulfate, filter, and spin the filtrate Most of the low-boiling point solvent was removed by evaporation, and then the product was distilled under reduced pressure to obtain 35 g (87%) of the product 2-methyl-1-hexanol (166-167° C. / 760 mm). The chemical purity measured by gas chromatography was >98%.
[0025] (2) At 0°C, 25.07 grams of methanesulfonyl chloride ( 0.22mol, 16.94mL), the dropwise addition was completed within 10 to 15 minutes, then stirred for 30 minutes, poured into ice water, separated in...
Embodiment 2
[0029] (1) At 0°C, after mixing 58.08 grams (1.00mol, 69.26mL) of allyl alcohol and 600mL of n-pentane, they were added dropwise to 1.2M n-butyllithium (2.50mol, 2083mL) within 30 minutes, and stirred at room temperature React for 1 hour. At 0°C, carefully add 600 mL of water to the reaction system, stir the reaction for 30 minutes, separate the liquids, wash the organic layer with 260 mL of 1M hydrochloric acid, then wash twice with 90 mL of water, dry the organic layer with anhydrous magnesium sulfate, filter, and the filtrate is rotovapped Most of the low-boiling point solvent was removed, and the residue was distilled under reduced pressure to obtain 98.8 g (85%) of the product 2-methyl-1-hexanol (166-167° C. / 760 mm). The chemical purity measured by gas chromatography was >98%.
[0030] (2) At 0°C, add 80 mL of dichloromethane and p-methanol dropwise to a mixed solution of 23.2 grams (0.2 mol) of 2-methyl-1-hexanol, 150 mL of dichloromethane, and 42 mL (0.3 mol) of trieth...
Embodiment 3
[0034] (1) At 0°C, add 108.28 g (0.40 mol) of phosphorus tribromide dropwise to 116.2 g (1.0 mol) of 2-methyl-1-hexanol, and the dropwise addition is completed within 30 minutes, then stir at room temperature for 15 hours , the reactant was poured into ice water, separated into layers, and the water layer was removed. The organic layer was washed twice with 5 mL of concentrated sulfuric acid, washed twice with 20 mL of water, and washed with 20 mL of saturated sodium carbonate solution. The organic layer was dried with anhydrous magnesium sulfate and filtered. The residue was distilled under reduced pressure to obtain 89.55 g (50%) of the product 2-methyl-1-bromohexane.
[0035] (2) 13.72 g (0.4 mol) of 70% sodium hydride was washed with toluene to remove mineral oil, put in a flask, added 400 ml of toluene, and 64.03 g (0.4 mol, 60.70 mL) of diethyl malonate was added dropwise with stirring ), the reaction emits hydrogen, until no gas is released, add phase transfer catalyst ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com