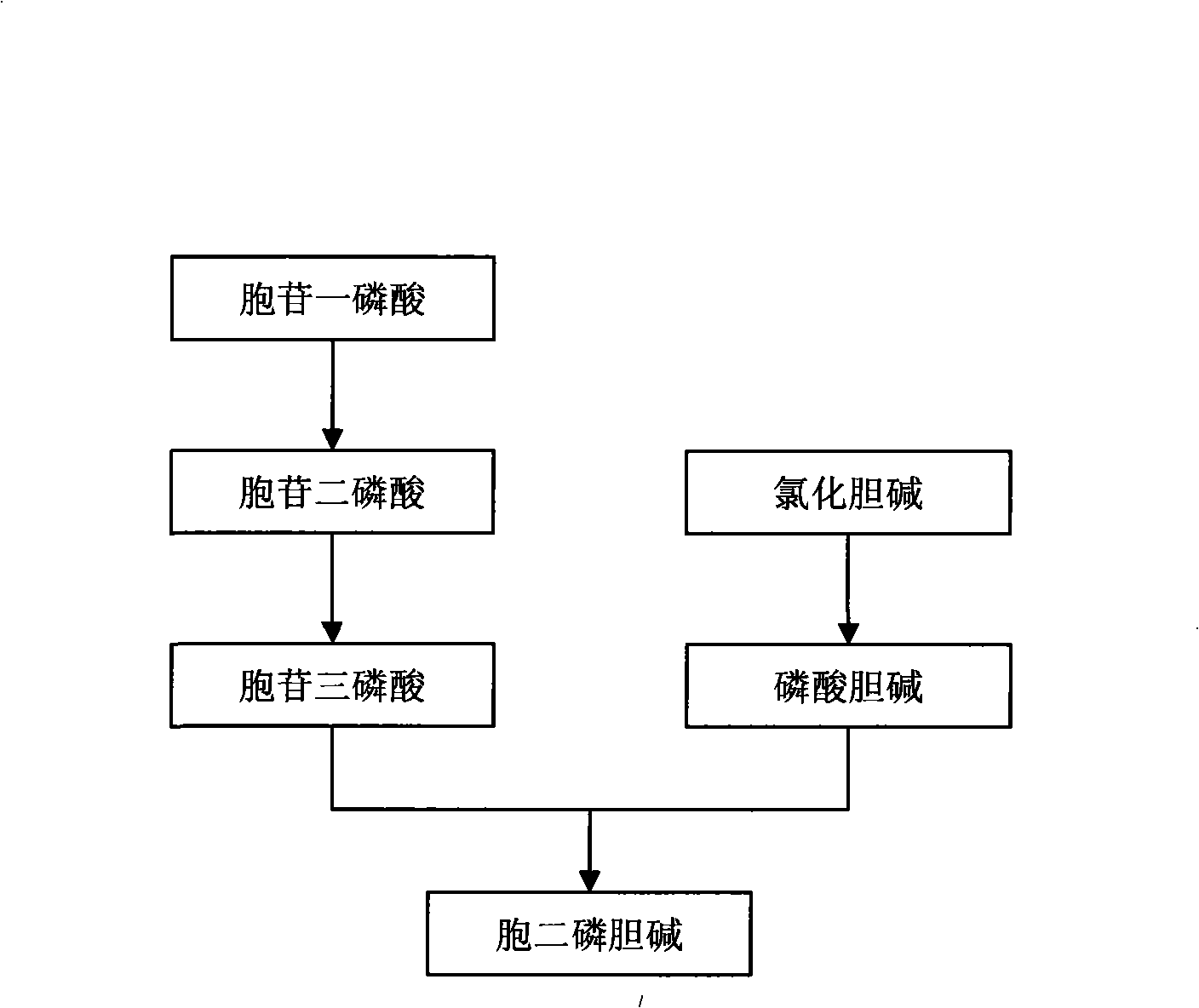
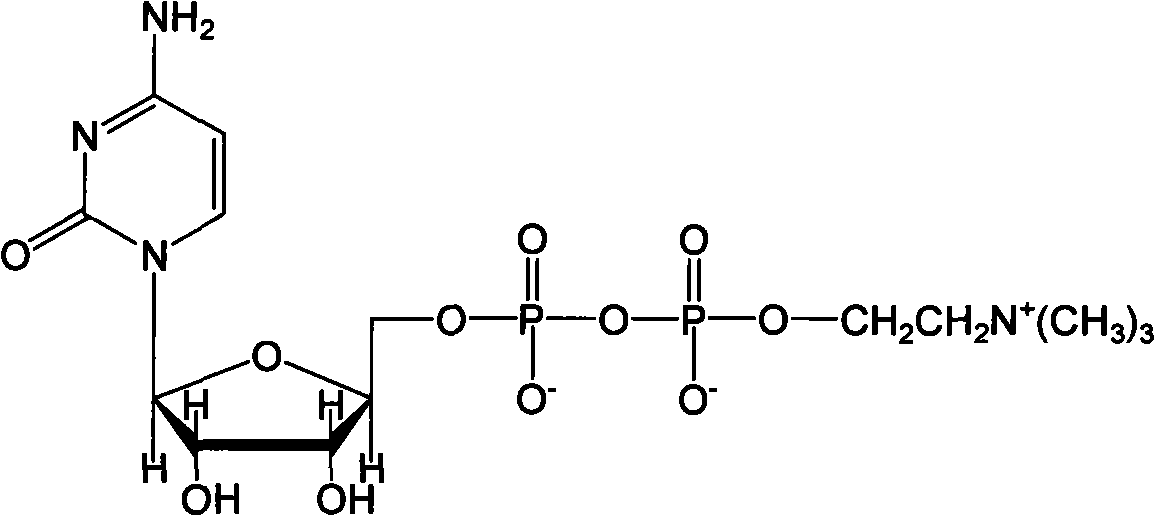
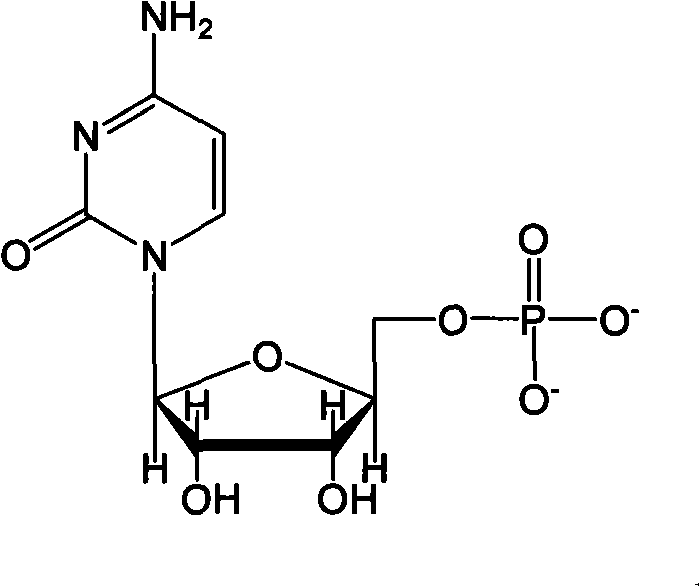
Method for preparing citicoline
A technology for citicoline and choline chloride, applied in the field of preparation of citicoline, can solve the problems of increased operation intensity, complicated process, increased equipment and the like, and achieves improved utilization rate, high conversion efficiency and cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Yeast medium (g / L): glucose 40, urea 2.0, potassium dihydrogen phosphate 1.5, magnesium sulfate heptahydrate 0.5, zinc sulfate heptahydrate 4.0×10 -3 , ferrous sulfate heptahydrate 3.0×10 -3 , manganese chloride tetrahydrate 0.3×10 -3 , anhydrous calcium chloride 1.0×10 -3 , Biotin 0.05×10 -3 . Saccharomyces cerevisiae inoculum was 10%, cultured on a shaker at 120 rpm at 30°C for 24 hours, and centrifuged at 4000 rpm for 20 minutes. Take the yeast paste and store it at -7°C for later use.
Embodiment 2
[0045] Embodiment 2: Using CMP and choline chloride as substrates and glucose as energy donor to prepare citicoline
[0046] In a reaction tank with a capacity of 15L, prepare 60mM choline chloride, 0.20M sodium dihydrogen phosphate, 30mM CMP, 0.3M glucose, 50mM magnesium sulfate, 1mM potassium chloride, 2mM cysteine, using Example 1 2800 grams of Saccharomyces cerevisiae mud, 10 grams of cetyltrimethylamine ammonium bromide and 10 L of reaction liquid cultured by the described method were used to adjust the pH to 6.8 with sodium hydroxide, and stirred at a low speed for 10 hours at 37 ° C. , after the reaction, centrifugal precipitation, supernatant is carried out citicoline quantitative analysis, contains citicoline 14.9 grams per liter in the conversion liquid, and the conversion rate of citicoline reaches 97.4% (mol meter) .
Embodiment 3
[0047] Example 3: Preparation of Citicoline with Cytidine and Choline Chloride as Substrates and Glucose as Energy Donor
[0048] In a reaction tank with a capacity of 15L, prepare 150mM choline chloride, 0.5M potassium dihydrogen phosphate, 50mM cytidine, 0.5M glucose, 2mM magnesium sulfate, 5mM mannitol, and utilize the method described in Example 1 to cultivate 2700g of baker's yeast puree, 10g of Triton X-100 and 12L of reaction liquid, adjust the pH to 6.0 with sodium hydroxide, and stir at 32°C for 15 hours at a low speed. Quantitative analysis of citicoline was carried out, the conversion liquid contained citicoline 22.95 g / liter, and the conversion rate of citicoline reached 90% (in mol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com