Method for separating and extracting isostearic acid from monomer acid
A technology of isostearic acid and isostearate, which is applied in the field of separation and extraction of isostearic acid, can solve problems such as low antioxidant performance, high iodine value, and easy darkening of product color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
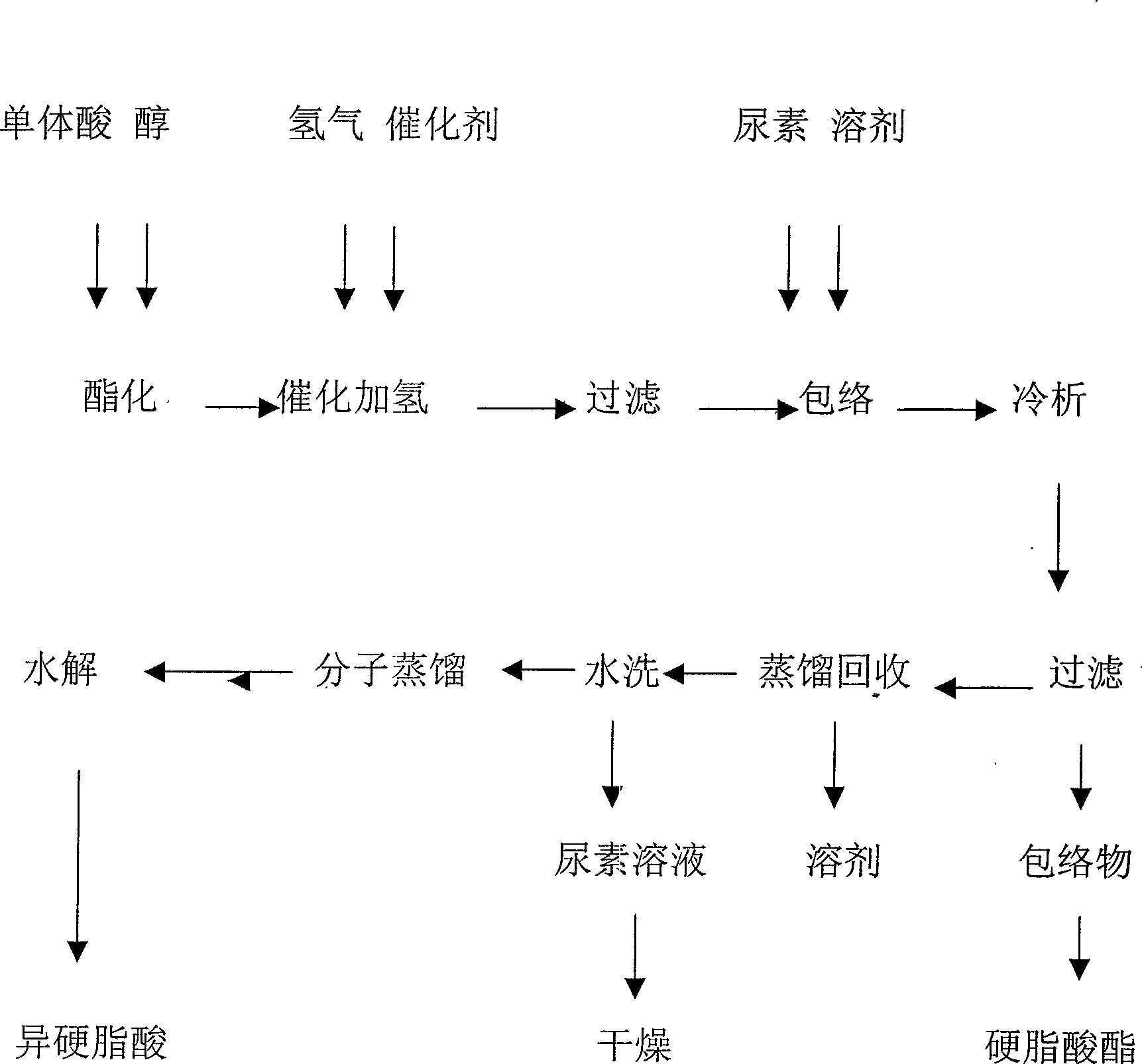
[0022] A method for separating and extracting isostearic acid from monomeric acids, comprising the following steps:
[0023] (1) Esterification of monomers
[0024] The monomeric acid and anhydrous methanol are pumped into the pipeline reactor with a pressure pump at a volume ratio of 1:1, and the feed of the monomeric acid and methanol is maintained at a pressure of 2.0MPa and a reaction temperature of 160-240°C The speed is 50L / h, react for 19 hours, when the acid value of the reactant drops below 2mgKOH / g, the methyl esterification reaction is completed, vacuumize, and distill off water and methanol at 0.07MPa and a temperature of 35-65°C to obtain monomeric acid methyl ester. Methanol can be reused after dehydration.
[0025] (2) Catalytic hydrogenation of monomeric acid ester
[0026] Add the monomeric acid methyl ester obtained in (1) into the hydrogenation reactor, drop into the Raney nickel catalyst of 0.3% of the monomeric acid weight, seal the reactor, vacuumize, ...
Embodiment 2
[0034] A method for separating and extracting isostearic acid from monomeric acids, comprising the following steps:
[0035] (1) Esterification of monomers
[0036] Pump the monomeric acid and absolute ethanol into the pipeline reactor with a pressure pump at a ratio of 1:1.1 by volume, and keep the feed of monomeric acid and ethanol under the conditions of 2.5MPa pressure and reaction temperature of 170-250°C The speed is 45L / h, react for 20 hours, when the acid value of the reactant drops below 2mgKOH / g, the esterification reaction is completed, vacuumize, and distill off ethanol under the conditions of 0.06MPa and temperature 50-70℃ to obtain monomeric acid ethyl ester. Recycled ethanol can be reused after dehydration.
[0037] (2) Catalytic hydrogenation of monomeric acid ethyl ester
[0038] Put the product obtained in (1) into the hydrogenation reaction kettle, put into the Raney nickel catalyst of 0.3% by weight of the monomeric acid, seal the reaction kettle, vacuum...
Embodiment 3
[0046] A method for separating and extracting isostearic acid from monomeric acids, comprising the following steps:
[0047] (1) Esterification of monomers
[0048] Put the monomeric acid and anhydrous isopropanol into the pipeline reactor with a pressure pump at a ratio of 1:1.1 by volume, and keep the monomeric acid and isopropyl alcohol under the conditions of 3.0MPa pressure and reaction temperature of 160-240°C The feed rate of alcohol is 40L / h, react for 21 hours, when the acid value of the reactant drops below 2mgKOH / g, the esterification reaction is completed, vacuumize, and distill and remove isopropyl under the conditions of 0.07MPa and temperature 50-70℃ alcohol to obtain isopropyl monomeric acid.
[0049] (2) Catalytic hydrogenation of monomeric acid ester
[0050] Add the monomeric acid isopropyl ester obtained in (1) into the hydrogenation reactor, drop into the Raney nickel catalyst of 0.3% by weight of the monomeric acid isopropylate, seal the reactor, and va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Saponification value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com