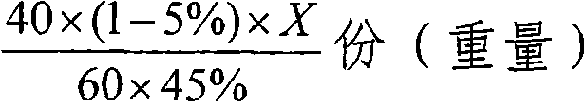Method for preparing cryolite and coproducing soluble glass by using hydrof luorosilicic acid
A cryolite, fluorosilicic acid technology, applied in the directions of silicate, alkali metal silicate, aluminum fluoride, etc., can solve the problems of difficult recycling, serious environmental pollution, high cost of raw materials, and achieve easy implementation and application, high purity High and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
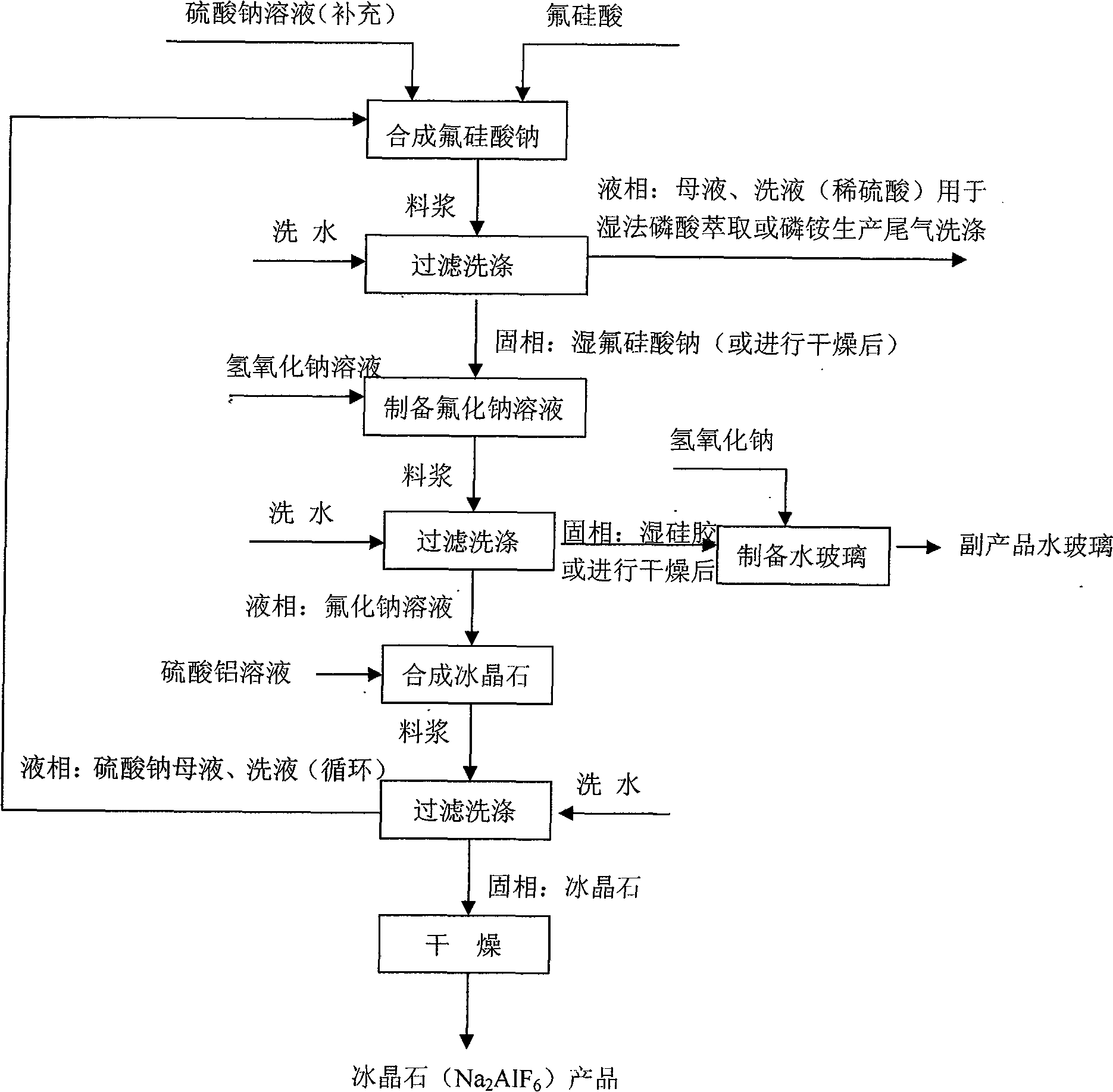
[0030] (1) Synthesis of sodium fluorosilicate: 10% wet-process phosphoric acid by-product fluosilicic acid solution is added with 28% sodium sulfate solution under stirring, and the sodium sulfate addition is 110% of the theoretically calculated amount, and the feeding time is 30 minutes. After the material is finished, continue to stir and react for 20 minutes, and sodium fluorosilicate crystals are precipitated to generate dilute sulfuric acid mother liquor. The slurry is filtered, and the filter cake is washed with 5% dilute sodium carbonate solution to obtain sodium fluorosilicate with a free water content of 7%, which is ready for use.
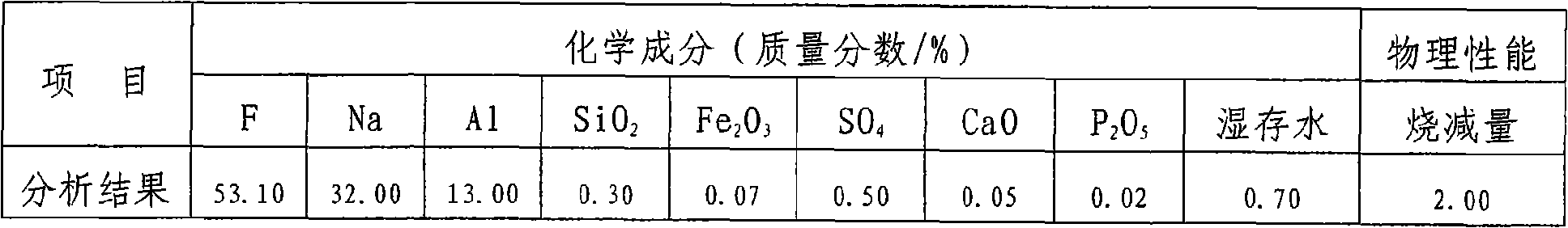
[0031] (2) Preparation of sodium fluoride solution and water glass: 42% liquid sodium hydroxide, heated to 92°C, under constant stirring, slowly add the sodium fluorosilicate obtained in (1), maintain the reaction temperature at 92°C, Under alkaline conditions, the stirring reaction was carried out for 60 minutes, and the terminal alkalin...
Embodiment 2~6
[0038]Repeat the same steps as described in Example 1, but in the step of synthesizing sodium fluorosilicate, the concentration of fluorosilicic acid used is respectively 5%, 8%, 15%, 20% or 25%, and the amount of sodium sulfate added They are respectively 105%, 115%, 120%, 125% or 130% of the theoretically calculated amount, and the addition reaction time is respectively 30 minutes, 45 minutes, 60 minutes, 75 minutes and 90 minutes. The control parameters of preparing sodium fluoride solution and water glass step and synthesizing cryolite step are identical with embodiment 1.
Embodiment 7
[0040] Technological process is identical with embodiment 1, only concrete processing condition is:
[0041] The phosphate fertilizer by-product fluosilicic acid with a concentration of 5% is stirred and reacted with sodium sulfate solution at room temperature, the concentration of the recycled sodium sulfate mother liquor is 3%, the concentration of the supplemented sodium sulfate solution is 25%, and the total amount of sodium sulfate added is theoretically calculated The amount is 100%, the synthesis time is 90 minutes, and the sodium fluorosilicate slurry is obtained, and the sodium fluorosilicate slurry is separated from liquid to solid, and the filter cake is washed with normal temperature water to obtain sodium fluorosilicate with a moisture content of 9%, and dried to After the moisture content is lower than 0.3%, it is ready for use. Use 25% liquid caustic soda or make a 25% solution with solid caustic soda, preheat to 85°C, and slowly add the above-mentioned dried so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com