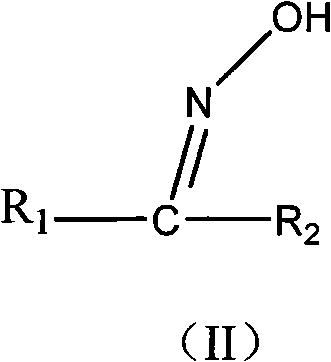
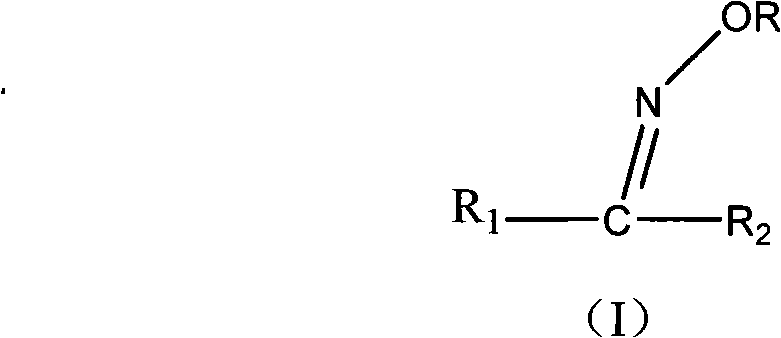Method for synthesizing oxime ether
A technology of oxime ether and ether, applied in the field of organic intermediate synthesis, can solve problems such as complicated operation, hidden danger, complicated process, etc., and achieve the effects of improving the operating environment, low investment, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 26g of [Bmim]BF to a 100mL flask equipped with an electric stirrer, a thermometer, and a condenser 4 , 8.7g (0.10mol) of 99% methyl ethyl ketone oxime, 4.4g99% NaOH (0.11mol), the outside of the flask was cooled with a water bath, and then 10.9g (0.10mol) bromoethane was added dropwise at 20°C, Add dropwise for 1h, and keep warm for 1h after the dropwise addition. The reaction solution was sampled and extracted with ether and then analyzed by gaseous gas. The result was 0.3% of methyl ethyl ketone oxime, 1.8% of bromoethane, 95.6% of O-ethyl methyl ethyl ketone oxime, and 2.3% of other impurities. The reaction solution was filtered, and the filter residue (salt content) was drained as much as possible. The filter residue was washed with 20 g of diethyl ether in three equal amounts, the filter residue was discarded, and the diethyl ether wash was used for extraction; the reaction solution after filtering to remove salt was extracted with 70 g (including 20 g of diet...
Embodiment 2
[0031] The 24.6g of embodiment 1 is reused [Bmim] BF 4 Replace newly added [Bmim] BF4, other with embodiment 1, the result is methyl ethyl ketoxime 0.3%, bromoethane 1.9%, O-ethyl methyl ethyl ketone oxime 95.7%, other impurity 2.2%. 9.6 g of the product O-ethylmethyl ethyl ketone oxime was obtained, the yield was 83.5%, and the product purity was 98.2% by gas phase detection.
Embodiment 3
[0033] Bromoethane is changed into 13.1g ((0.12mol), NaOH is 5.2g (0.13mol), other is the same as embodiment 1, the result is methyl ethyl ketoxime 0.2%, bromoethane 3.2%, O-ethyl methyl Ethyl ketone oxime 94.5%, other impurities 2.1%, yield 78.4%, gas phase detection product purity 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com