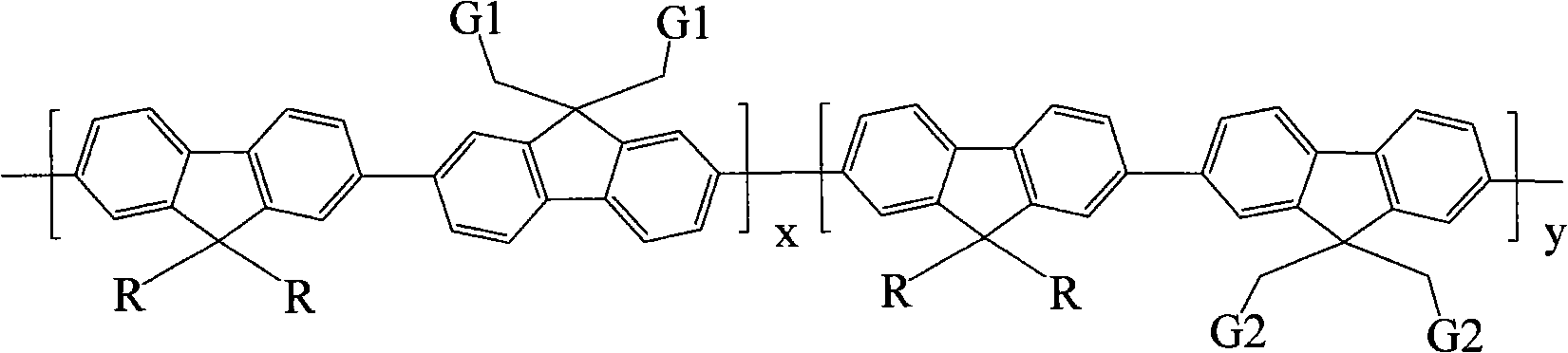
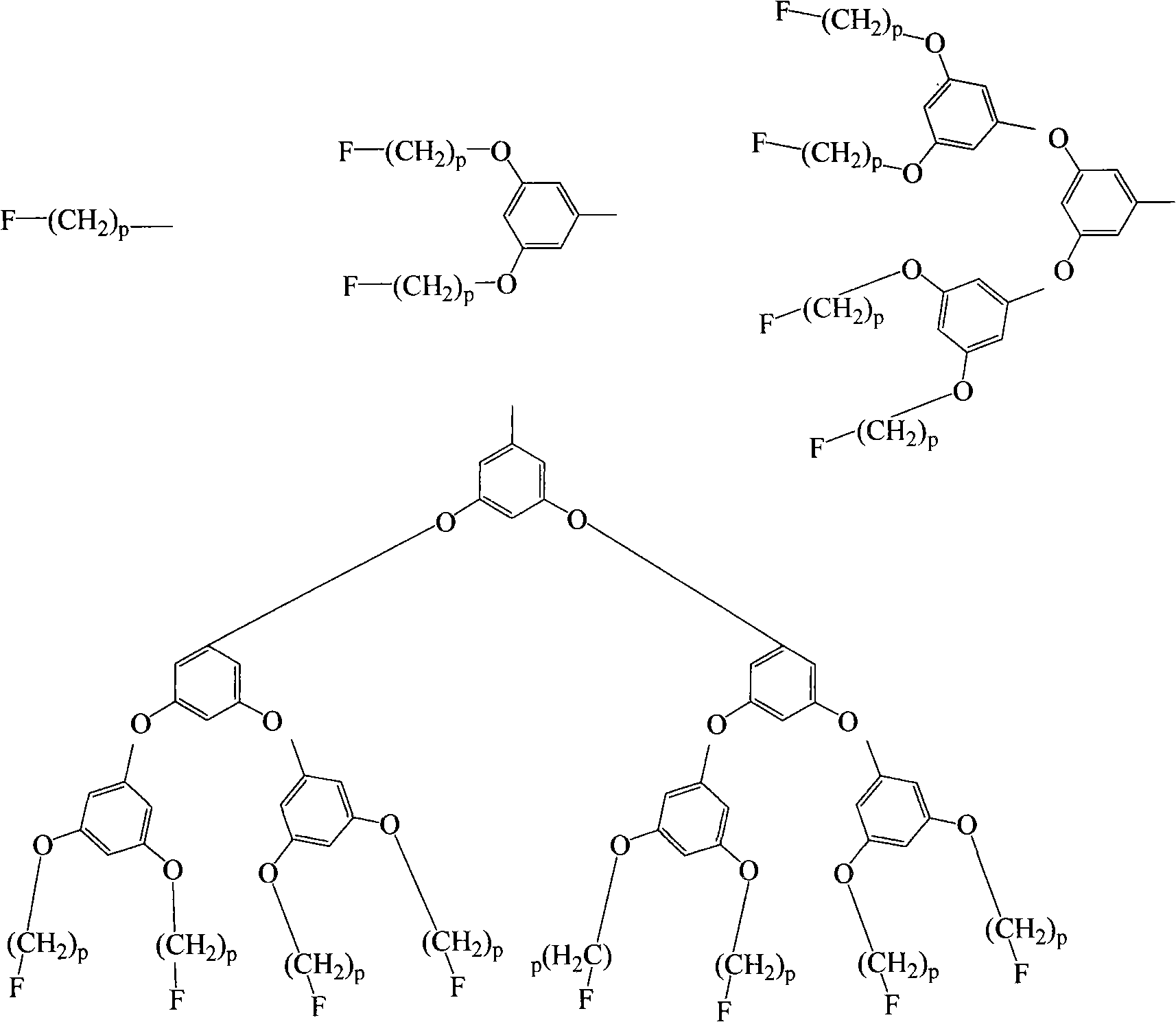
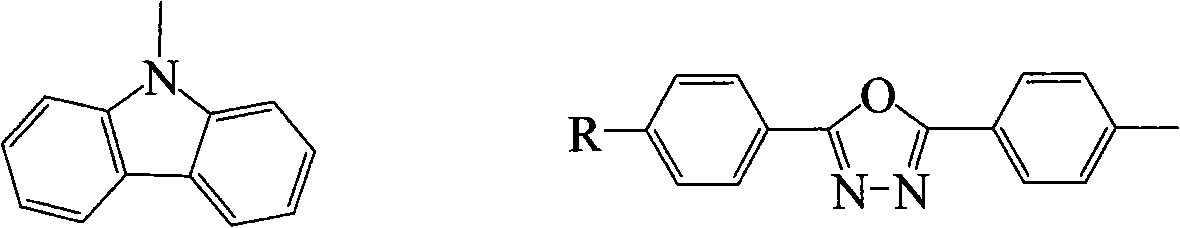
Arborization functional group side chain-containing polyfluorene macromolecule blue photoelectric material and application thereof
A functional group, polyalkyl fluorene technology, applied in the field of polyalkyl fluorene polymer blue optoelectronic materials, can solve the problems of red shift of emission wavelength, affecting the purity of emission color, etc., to reduce the chance of oxidation and improve holes Injection and transmission performance, effect of suppressing long-wave emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of Intermediate 1
[0032] In a 100 ml three-necked flask, add 5.16 g (15.6 mmol) 9-(6-bromohexyl) carbazole, 1.00 g (7.1 mmol) 3,5-dihydroxybenzyl alcohol, 3.68 g (65 mmol) Anhydrous potassium carbonate, 0.38 g (1.42 mmol) of 18 crown 6 and 60 ml of anhydrous acetone form a mixed solution. The reaction mixture was stirred and refluxed under nitrogen atmosphere for 60 hours, and then cooled to room temperature naturally. The solvent was distilled off under reduced pressure, and the solid residue was extracted with a mixed solvent of dichloromethane and water. The organic phases were combined, dried over anhydrous magnesium sulfate, and filtered. The primary product was separated by column chromatography (dichloromethane as the eluent) to obtain a pure product with a yield of 85%. 1 HNMR (CDCl 3 , 300MHZ, δ / ppm): 8.21(d, 4H), 7.47-7.41(m, 8H), 7.35(s, 1H), 7.25-7.20(m, 4H), 6.51(s, 2H), 6.26(s , 1H), 4.66(s, 2H), 4.39(t, 4H), 3.88(t, 4H), 2.10-2.06(m, 8H), ...
Embodiment 2
[0034] Synthesis of Intermediate 2
[0035] In a 50 mL round bottom flask, add 2.79 g (4.38 mmol) of intermediate 1, 2.90 g (8.75 mmol) of carbon tetrabromide, 2.29 g (8.75 mmol) of triphenylphosphine and 25 mL of anhydrous dichloro methane. The reaction mixture was stirred at room temperature for 5 hours under an argon atmosphere, and then cooled to room temperature. The solvent was evaporated under reduced pressure. The solid residue was separated by column chromatography using dichloromethane as the eluent to obtain a light yellow pure product with a yield of 90%. 1 HNMR (CDCl 3 , 300MHZ, δ / ppm): 8.16(d, 4H), 7.49-7.41(m, 8H), 7.22-7.19(m, 4H), 6.47(s, 2H), 6.30(s, 1H), 4.38-4.21 (m, 6H), 3.86(t, 4H), 2.10-2.08(m, 8H), 1.84-1.82(m, 8H).
Embodiment 3
[0037] Synthesis of intermediate 3
[0038] In a 100 ml three-necked flask, add 0.154 g (0.48 mmol) of 2,7-dibromofluorene, 2.78 mg (0.01 mmol) of tetrabutylammonium chloride and 60 ml of dimethyl sulfoxide to form a mixed solution, and pass argon After deoxygenation for 10 minutes, 2 ml of 50% sodium hydroxide solution was added. A mixed solution containing 0.718 g (1 mmol) of intermediate 2 in 80 ml of dimethyl sulfoxide was added dropwise to the above solution with stirring. After the reaction mixture was stirred at reflux under nitrogen atmosphere for 8 hours at room temperature, water was added to quench the reaction. The mixture was extracted three times with dichloromethane, and the organic phases were combined, dried over anhydrous magnesium sulfate, and filtered. The primary product was separated by column chromatography (dichloromethane:petroleum ether=4:1 as eluent) to obtain a pure product with a yield of 80%. 1 HNMR (CDCl 3 , 400MHz, δ / ppm): 8.12(d, 8H), 7.53(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com