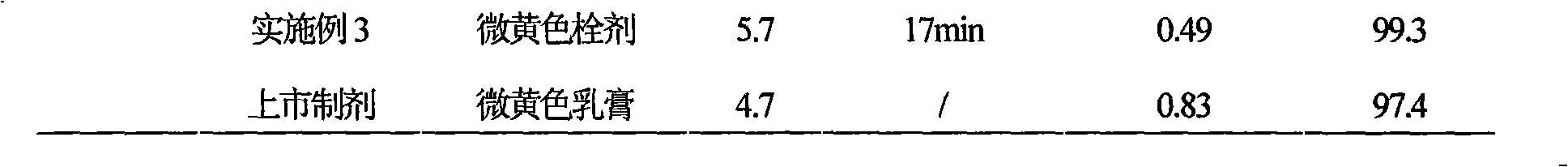Ciclopirox olamine pessary and preparation method thereof
A technology of ciclopirox olamine and vaginal suppositories, which is applied in ciclopirox olamine vaginal suppositories and its preparation, and the application field of medicines, can solve the problem of no longer considering the systemic absorption of medicines, affecting the dosage and contact time of medicines, and unrealized Zero-order release and other issues, to achieve good results, reduce the production of inflammatory mediators, easy to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
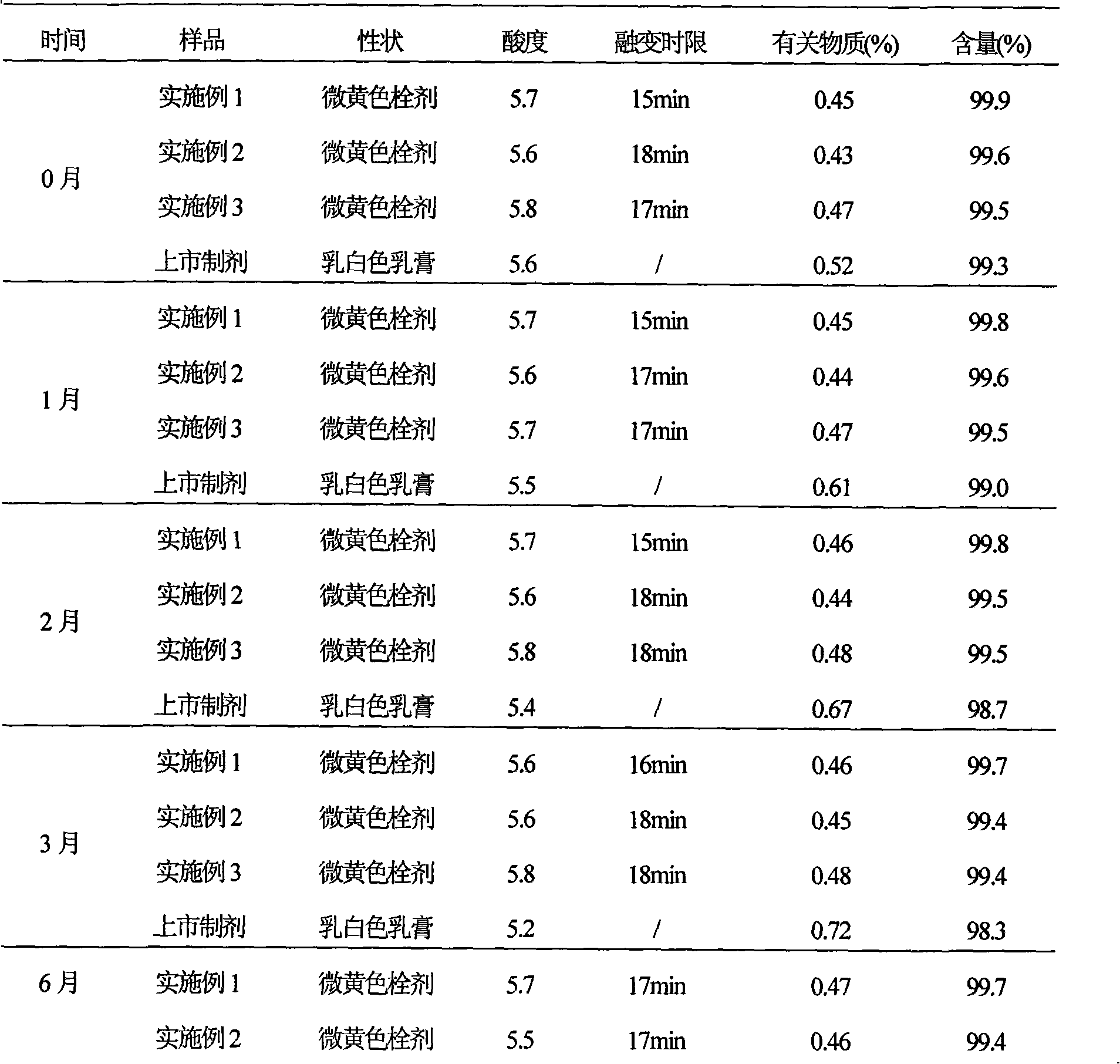
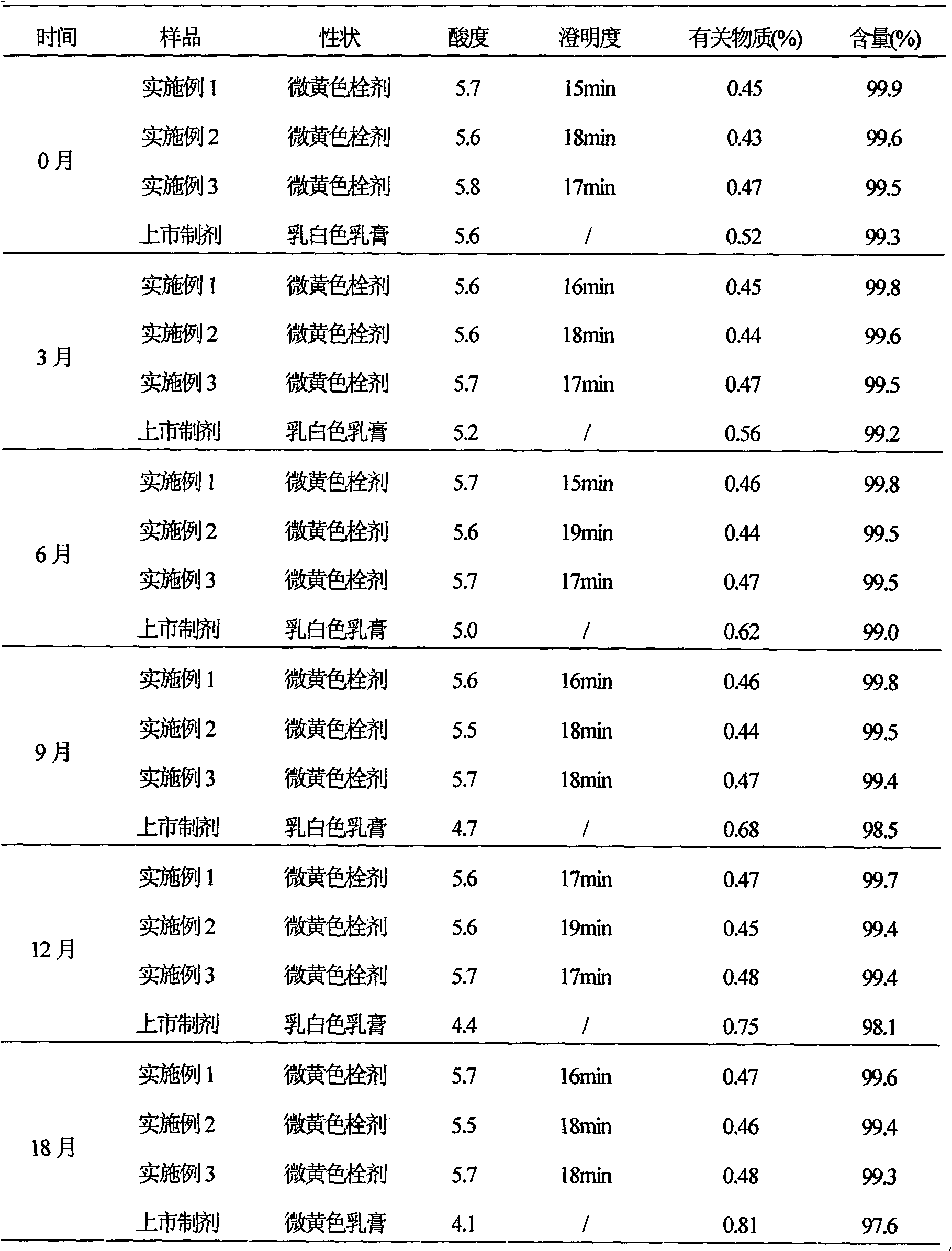
[0044] Example 1 Preparation of Ciclopirox vaginal suppository
[0045] Prescription (100 sticks)
[0046] Ciclopirox 10g
[0047] Poloxamer 188 700g
[0048] Stearic acid 5g
[0049] Hydrogenated castor oil 30g
[0050] Sodium benzoate 1g
[0051] Preparation Process:
[0052] (1) Crush 10g of ciclopirox and pass through a No. 6 sieve for use;
[0053] (2) Heat 700g poloxamer 188, 5g stearic acid, 30g hydrogenated castor oil and 1g sodium benzoate in a water bath until it melts completely, add 10g ciclopirox amine passing through No. 6 sieve, keep the temperature at 35°C and stir for 30min, Then pour it into the cooled and lubricant-coated suppository mould until it overflows the mould opening slightly;
[0054] (3) Cool down, control the temperature at 10°C, and completely solidify, scrape off the overflow part with a knife, open the model, push out the suppository, dry it, and pack to obtain the ciclopirox vaginal suppository.
Embodiment 2
[0055] Example 2 Preparation of Ciclopirox vaginal suppository
[0056] Prescription (100 sticks)
[0057] Ciclopirox 10g
[0058] Polysorbate 61 850g
[0059] Stearic acid 20g
[0060] Hydrogenated castor oil 2g
[0061] Sodium benzoate 10g
[0062] Preparation Process:
[0063] (1) Crush 10g of ciclopirox and pass through a No. 6 sieve for use;
[0064] (2) Heat 850g polysorbate 61, 20g stearic acid, 2g hydrogenated castor oil and 10g sodium benzoate in a water bath until it melts completely, add 10g ciclopirox amine passing through No. 6 sieve, keep at 40℃, stir for 20min, then Pour into the chilled and lubricant-coated suppository mold until it overflows the mold opening slightly;
[0065] (3) Cool down, control the temperature at 15°C, and completely solidify, scrape off the overflow part with a knife, open the model, push out the suppository, dry it, and pack to obtain the ciclopirox vaginal suppository.
Embodiment 3
[0066] Example 3 Preparation of Ciclopirox vaginal suppository
[0067] Prescription (100 sticks)
[0068] Ciclopirox 10g
[0069] Poloxamer 188 800g
[0070] Stearic acid 10g
[0071] Hydrogenated castor oil 15g
[0072] Sodium benzoate 5g
[0073] Preparation Process:
[0074] (1) Crush 10g of ciclopirox and pass through a No. 6 sieve for use;
[0075] (2) Heat 800g poloxamer 188, 10g stearic acid, 15g hydrogenated castor oil and 5g sodium benzoate in a water bath until it melts completely, add 10g ciclopirox amine passing through No. 6 sieve, keep at 37°C, stir for 20min, Then pour it into the cooled and lubricant-coated suppository mould until it overflows the mould opening slightly;
[0076] (3) Cool down, control the temperature at 8°C, make it completely solidified, cut off the overflow part with a knife, open the model, push out the suppository, dry it, and pack to obtain the ciclopirox vaginal suppository.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com