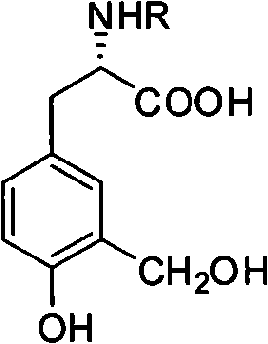
Synthesis of L-3-hydroxyl-4-methoxyl-5-methyl-phenylalaninol/phenylalanine
A compound and crude product technology, applied in the field of synthesis of L-3-hydroxy-4-methoxy-5-methyl-phenylalaninol/acid compound, can solve the problems affecting the yield of the synthesis process, the yield is not Ideal, expensive and other problems, to achieve the effect of easy industrialization, cheap reagents, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
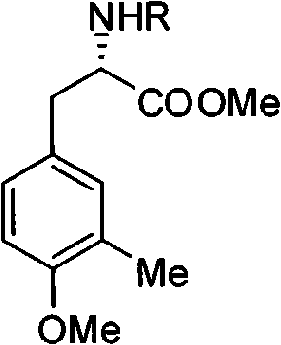
[0034] Embodiment 1: Compound five (R group is Cbz), namely the preparation of L-N-Cbz-3-methyl-4-methoxyl-phenylalanine methyl ester:
[0035] Add 1.62mmol of L-N-Cbz-3-hydroxymethyl-tyrosine (compound 4 whose R group is Cbz) into a 50mL round bottom flask, under the protection of argon, add 6.5mL of acetone, after the product dissolves, add 4.85mmol Potassium carbonate, 4.85mmol dimethyl sulfate, heated to reflux under magnetic stirring, reflux temperature 60°C, reacted under reflux conditions for 7h, TLC detected the reaction end point, after the reaction, distilled off acetone, added appropriate amount of water, extracted with ethyl acetate Three times, anhydrous Na 2 SO 4 After drying, the solvent was evaporated to obtain the crude product as light yellow liquid. Under argon protection, 14 mL of CH was added to the crude product 2 Cl 2 , then add 5.55mmol Et 3 SiH and 22.19 mmol CF 3 COOH, stirred at room temperature for 10h, after the reaction, dilute Na 2 CO 3 T...
Embodiment 2
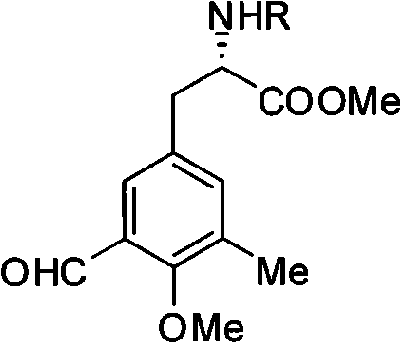
[0036] Embodiment 2: Preparation of compound six (R group is Cbz), i.e. L-N-Cbz-3-formyl-4-methoxy-5-methyl-phenylalanine methyl ester:
[0037] Add 1.362mmol of compound five (the R group is Cbz) in a 25mL round bottom flask, and add 5mL of CH 2 Cl 2 , then cool down to -10°C, add 3.268mmolTiCl 4 and 1.634 mmol Cl 2 CHOCH 3 , reacted for 3h, after the reaction, the reaction solution was poured into a beaker filled with ice water, stirred for 1h, extracted three times with ethyl acetate, anhydrous Na 2 SO 4 Drying and evaporating off the solvent gave the crude yellow-green liquid product, which was separated by a chromatographic column to obtain compound six (the R group is Cbz), with a yield of 89%; [α] D 26 +63(c 1.1, CHCl 3 );IR(neat)v max : 3314, 2954, 2866, 1748, 1692, 1541, 1259, 1214, 1058, 1006, 749, 698cm -1 ; 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 10.32 (1H, s), 7.42 (1H, s), 7.25-7.37 (5H, m), 7.19 (1H, s), 5.31 (1H, d, J = 8.0), 5.12 (1H , d, J=12.2), 5.07 ...
Embodiment 3
[0038] Embodiment 3: Preparation of compound seven (R group is Cbz), i.e. L-N-Cbz-3-hydroxyl-4-methoxyl-5-methyl-phenylalaninol:
[0039] Add 1.1mmol compound six (the R group is Cbz) and 11mL dichloromethane into a 25mL round bottom flask, add 1.65mmol m-chloroperoxybenzoic acid after the reactant is dissolved, stir at room temperature for 13h, then wash with dilute Na 2 CO 3 The solution was neutralized, extracted three times with dichloromethane, the organic phases were combined, washed with saturated NaCl solution, anhydrous NaCl 2 SO 4 Dry and evaporate the solvent to obtain a yellow liquid crude product, add THF to the crude product without treatment, the concentration is 0.1mol / L, add 2eq of LiBH 4 , After reacting for 2h, the solvent was distilled off under reduced pressure, extracted three times with ethyl acetate, anhydrous Na 2 SO 4 Drying and evaporating the solvent gave the yellow-green liquid crude product, which was separated by a chromatographic column to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com