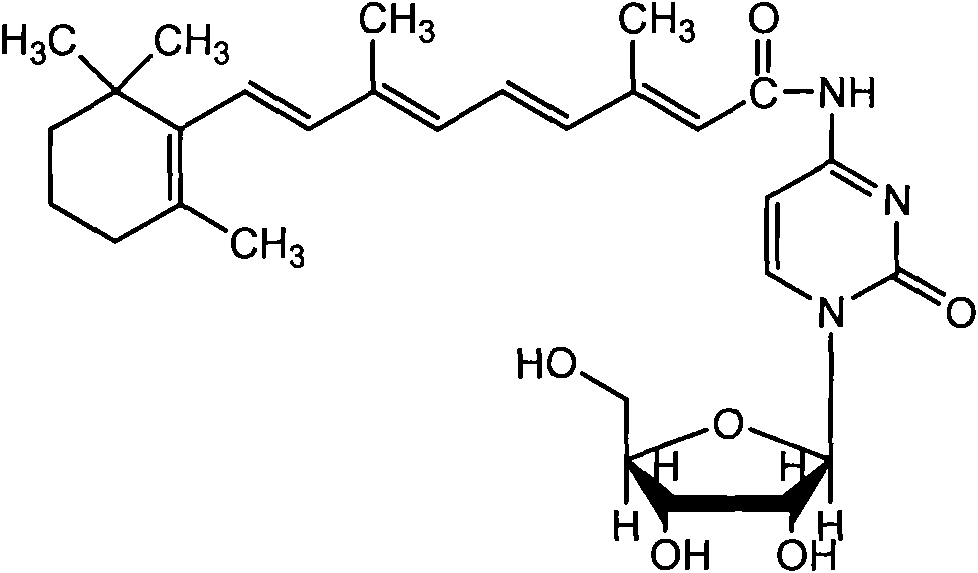
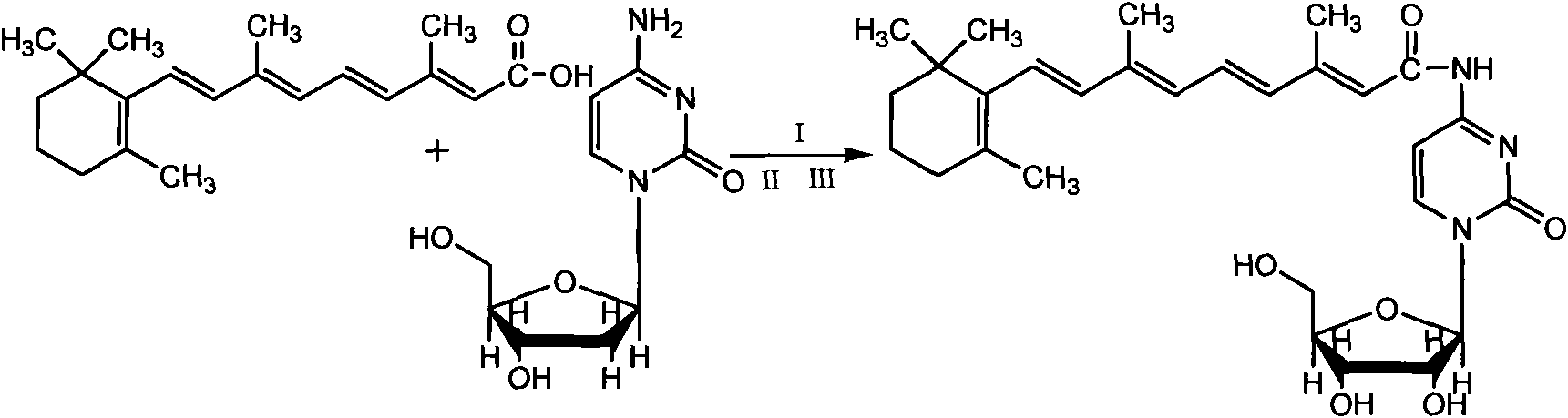
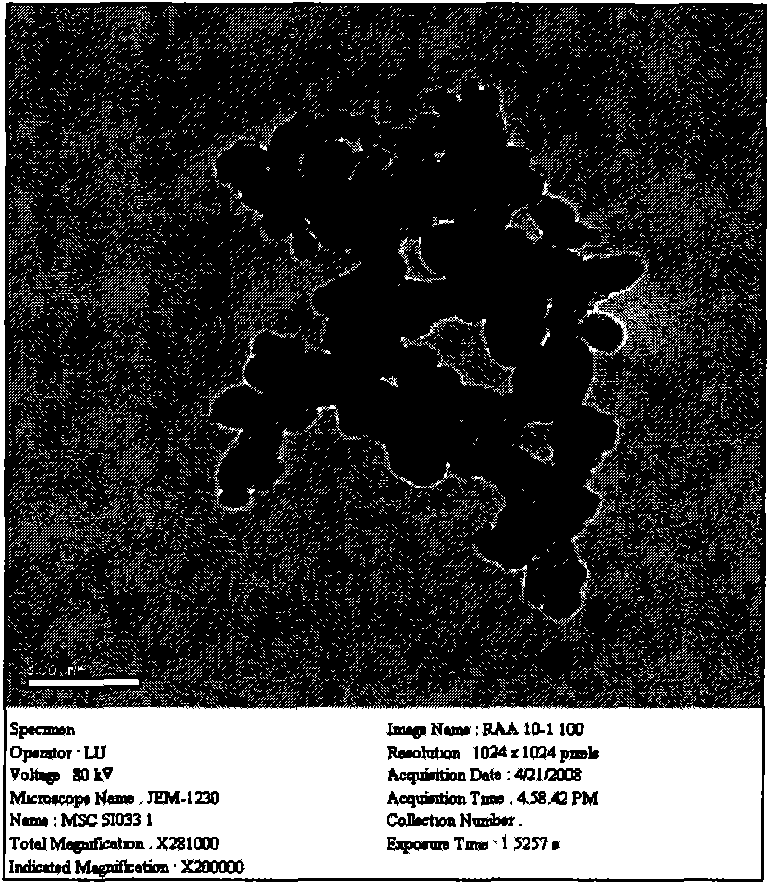
Vitamin A acyl cytosine arabinoside conjugate and pharmacome thereof as well as preparation methods and application thereof
A technology of retinyl cytarabine and said formyl arabinoside, which is applied in the field of biomedicine, can solve the problems of low encapsulation rate, no amphiphilicity, easy leakage and the like, and achieves the effect of protection and inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1, Preparation of Retinoyl Cytarabine Conjugate
[0026] Dissolve 0.6g (2mmol) ATRA (all-trans retinoic acid), 0.454g (2.2mmol) DCC (dicyclohexylcarbodiimide) and 0.270g (2mmol) HOBT (1-hydroxybenzotriazole) in Add 0.558g (2mmol) HCl·Ara-C (cytarabine hydrochloride) to an appropriate amount of anhydrous pyridine solution under the conditions of protecting from light and Ar gas, and then add 0.558g (2mmol) of HCl Ara-C (cytarabine hydrochloride) after stirring in an ice bath for 20 minutes. Protected, stirred at room temperature for 2 days, TLC (chloroform / methanol, 10:1) showed that the points of ATRA and Ara-C became shallower, the reaction solution was spin-dried under reduced pressure, added an appropriate amount of chloroform to dissolve, and filtered out DCU (dicyclohexyl urea ), the filtrate was spin-dried under reduced pressure, the residue was washed with excess ether, the ether was removed by rotary evaporation under reduced pressure, the residue was di...
Embodiment 2
[0027] The preparation of embodiment 2 retinoyl cytarabine drug plasmid
[0028] Weigh the retinoyl cytarabine conjugate prepared in Example 1 into an eggplant-shaped bottle, add an appropriate amount of lecithin and fully dissolve it with chloroform, place it on a rotary evaporator, and remove the solvent by rotary evaporation in a constant temperature water bath at 35°C until A layer of gel film appears on the wall of the bottle, add phosphate buffer, then vibrate by hand or on a vortex mixer until all or part of the film falls off, sonicate in a water bath for 20 minutes, and the target compound can be dissolved in phosphate buffer with the help of lecithin A uniform dispersion system is formed in the liquid, with no particles by visual inspection, and light blue scattered light. Under the optical microscope, some uniform small particles can be seen swimming, and it is preliminarily determined that a vesicle-shaped drug substance is obtained. Through the measurement of the...
experiment example 1
[0029] Experimental example 1 Inhibitory effect of retinoyl cytarabine conjugates on the proliferation of human leukemia cells HL-60
[0030] Test compound: retinoyl cytarabine conjugate (ATRA-Ara-C) prepared in Example 1;
[0031] Human leukemia cell line HL-60 was regularly subcultured with RPMI1640 culture medium (containing 10% inactivated fetal bovine serum, penicillin 100u / ml, streptomycin 100ug / ml).
[0032] Drug preparation: the positive control is all-trans retinoic acid (ATRA), cytarabine (Ara-C) and the physical mixture of all-trans retinoic acid and cytarabine (ATRA+Ara-C) dissolved in 1 ‰ ethanol in PBS phosphate buffered saline made 5 x 10 -6 mol / L concentration solution, the test compound was dissolved in 1‰ ethanol PBS phosphate buffer solution to make a solution with the same amount concentration as the positive control group. Dilute to 8×10 before use -9 mol / L, 4×10 -8 mol / L, 2×10 -7 mol / L, 1×10 -6 mol / L, 5×10 -6 mol / L five concentrations. The negativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com