Starch-based amphiphilic self-assembled carrier material, preparation method thereof and application of material
A carrier material and amphiphilic technology, which is applied in the field of starch-based amphiphilic self-assembled carrier materials and its preparation, can solve the problems of unfavorable protection of the functional activity of active substances, difficulty in achieving controlled drug release, cell damage, etc., and achieve good results. Effects of gastrointestinal pH responsiveness, enhanced bioavailability, and enhanced immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
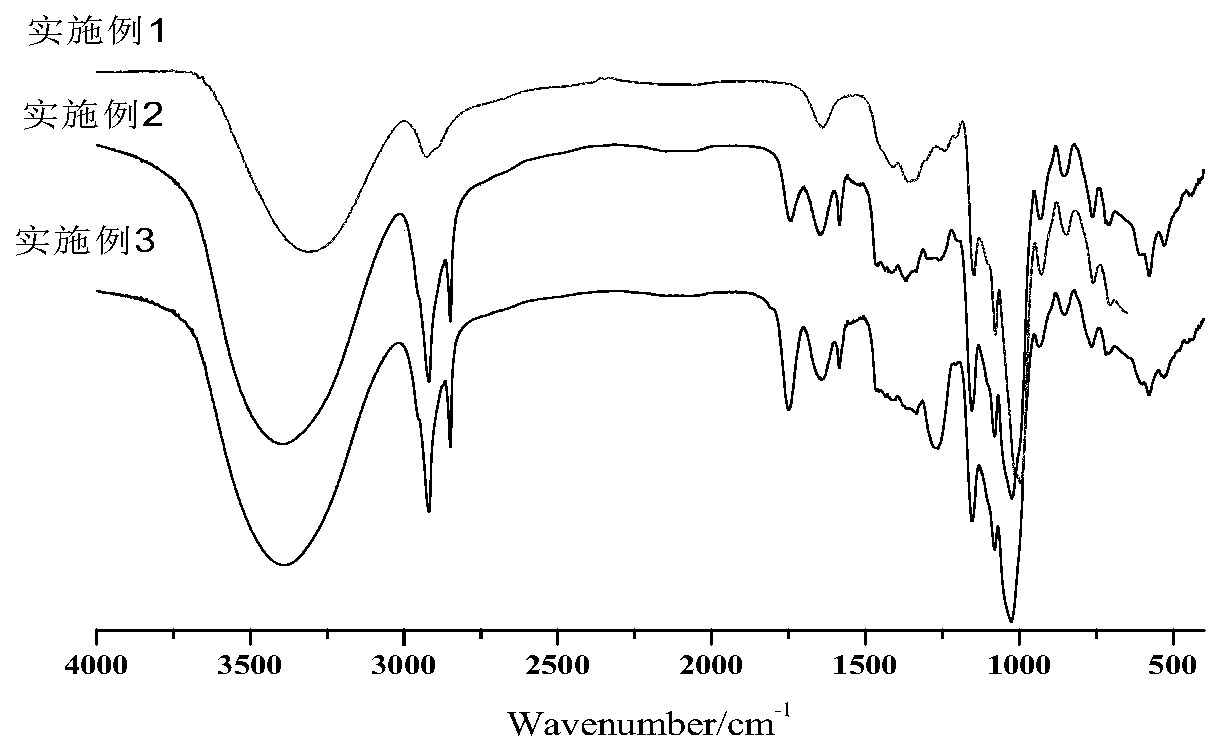
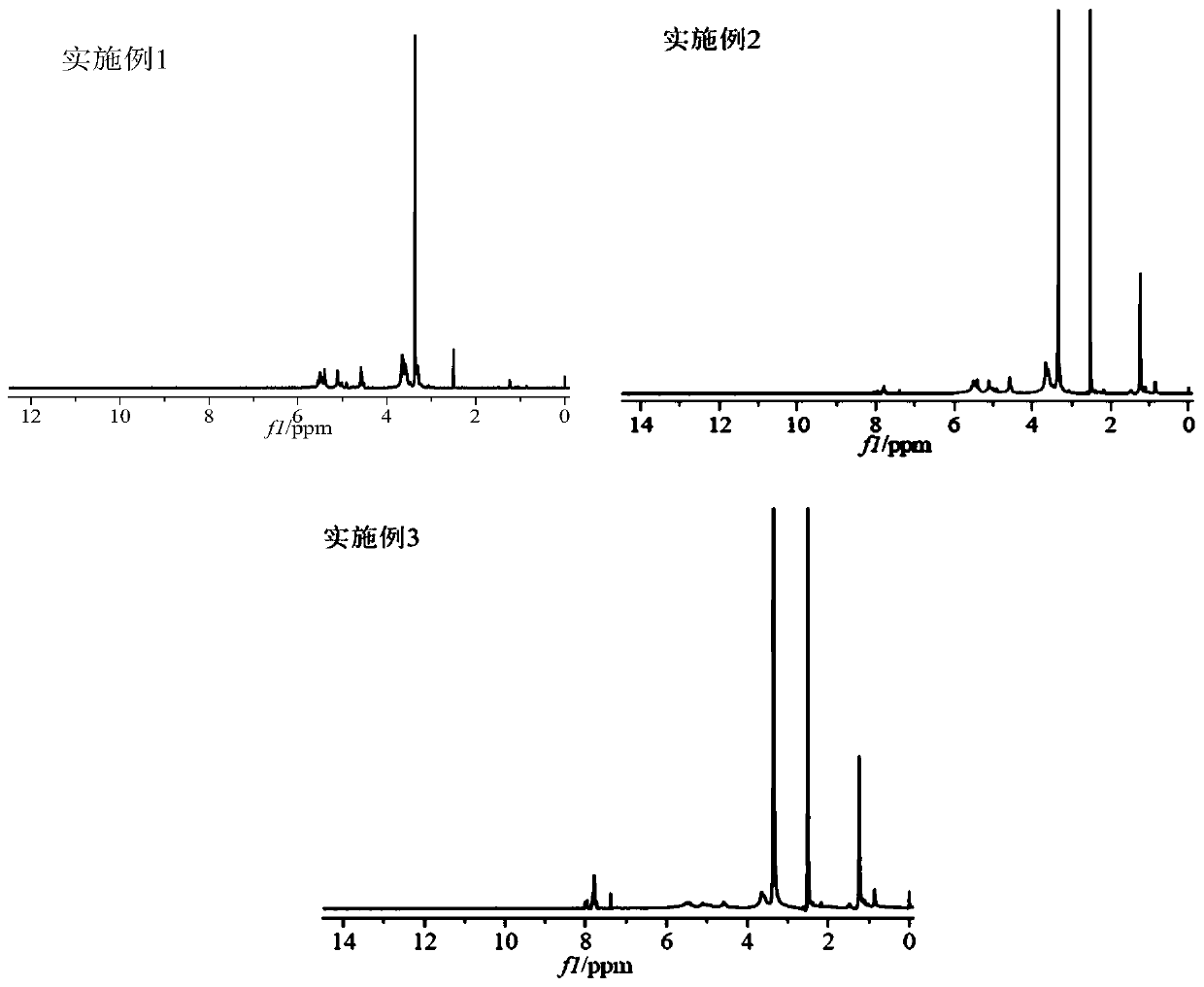
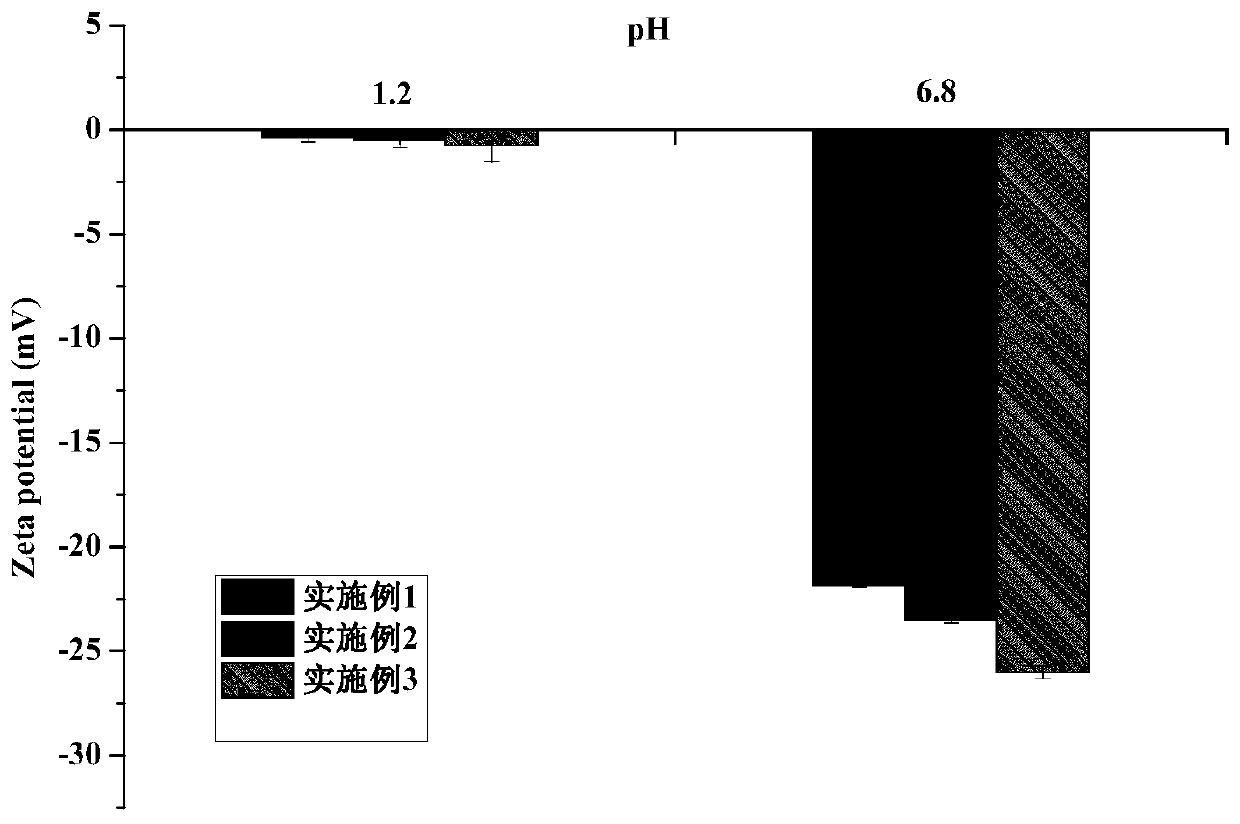
Image
Examples
Embodiment 1
[0063] (1) Starch (molecular weight is 1×10 6 g / mol) and monochloroacetic acid (starch: monochloroacetic acid=1:0.3, mol / mol) carry out etherification reaction 4h at 45 DEG C and obtain the carboxymethyl starch that carboxymethyl substitution degree is 0.20;
[0064] (2) With lauric acid (chain length is 12C) as raw material (starch: lauric acid=1:0.3, mol / mol), by DIC catalysis (DIC: fatty acid=1:1, mol / mol), with carboxymethyl The starch was reacted at 30°C for 24 hours to undergo an esterification reaction. After the reaction was completed, the amphiphilic starch whose hydrophobic side chain was lauric acid was obtained by dialysis and freeze-drying, and the degree of substitution of fatty acid was 0.21;
[0065] (3) Weigh 5g of amphiphilic starch whose hydrophobic side chain is lauric acid and dissolve it in 100mL of DMSO, add CDI with a molar ratio of starch:CDI=8:1, keep stirring at 15°C for 2h to activate starch hydroxyl groups, massage Il ratio starch:polypeptide=8:1,...
Embodiment 2
[0072] (1) Starch (molecular weight is 1×10 8 g / mol) and monochloroacetic acid (starch: monochloroacetic acid=1:0.4, mol / mol) are raw materials, carry out etherification reaction under 45 ℃ of reaction 4h, make carboxymethyl substitution degree be 0.27 carboxymethyl starch;
[0073] (2) With stearic acid (chain length is 18C) as raw material (starch: stearic acid=1:0.01, mol / mol), by DIC catalysis (DIC: fatty acid=1:1, mol / mol), at 30 Perform esterification reaction with carboxymethyl starch at ℃, and end the experiment after 24 hours of reaction. After dialysis and freeze-drying, an amphiphilic starch whose hydrophobic side chain is stearic acid is obtained, and the degree of substitution of fatty acid is 0.007;
[0074] (3) Weigh 2g of amphiphilic starch whose hydrophobic side chain is stearic acid and dissolve it in 100mL DMSO, add CDI with a molar ratio of starch:CDI=4:1, and continue stirring at 35°C for 4h to activate the starch hydroxyl groups, According to the molar ...
Embodiment 3
[0082] (1) Starch (molecular weight is 1×10 7 g / mol) and monochloroacetic acid (starch: monochloroacetic acid=1:0.4, mol / mol) are raw materials, carry out etherification reaction at 45 ℃, react 4h, make the carboxymethyl carboxymethyl substitution degree of 0.27 base starch;
[0083] (2) With stearic acid (chain length is 18C) as raw material (starch: stearic acid=1:0.5, mol / mol), by DIC catalysis (DIC: fatty acid=1:1, mol / mol), at 30 Perform esterification reaction with carboxymethyl starch at ℃, and finish the experiment after 24 hours of reaction. After dialysis and freeze-drying, an amphiphilic starch whose hydrophobic side chain is stearic acid is obtained, and the degree of substitution of fatty acid is 0.111;
[0084] (3) Weigh 6g of amphiphilic starch whose hydrophobic side chain is stearic acid and dissolve it in 100mL DMSO, add CDI with a molar ratio of starch:CDI=6:1, and keep stirring at 30°C for 3h to activate the starch hydroxyl groups, The corresponding mass o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com