Method for preparing special mirabilite with high purity
A manufacturing method and high-purity technology, applied in magnesium hydroxide and other directions, can solve the problems of agglomeration, inconvenience in use, and low product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
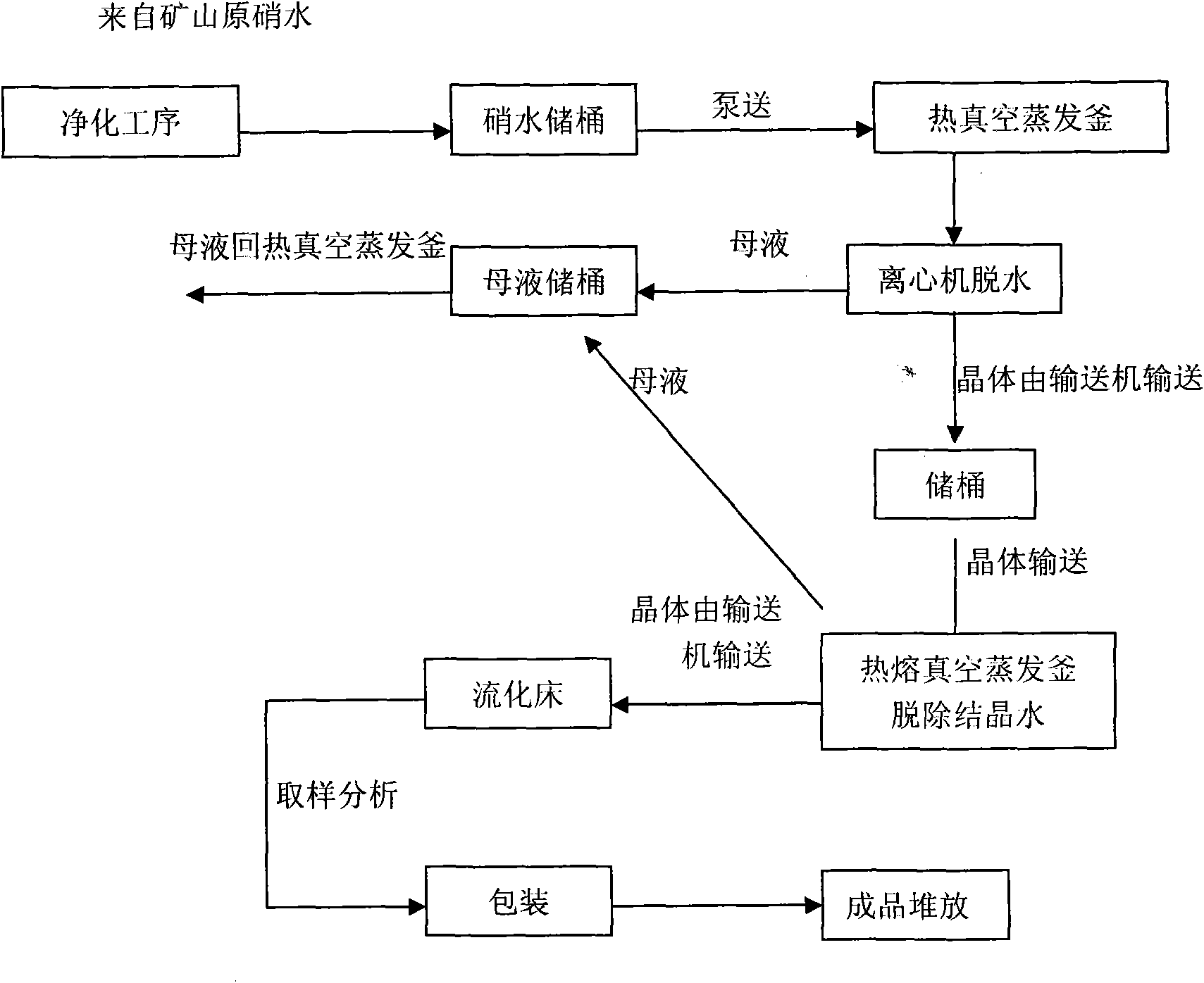
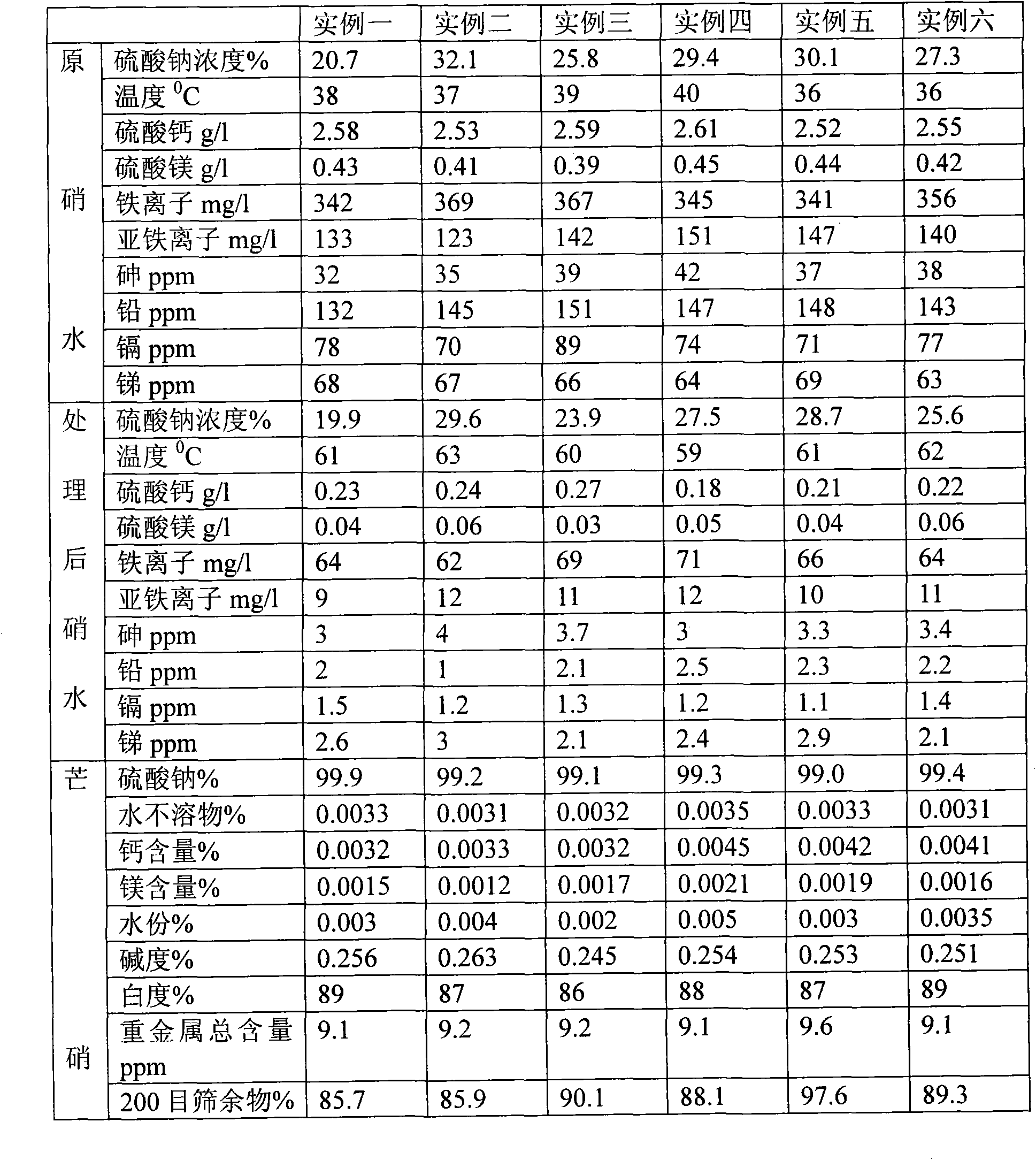
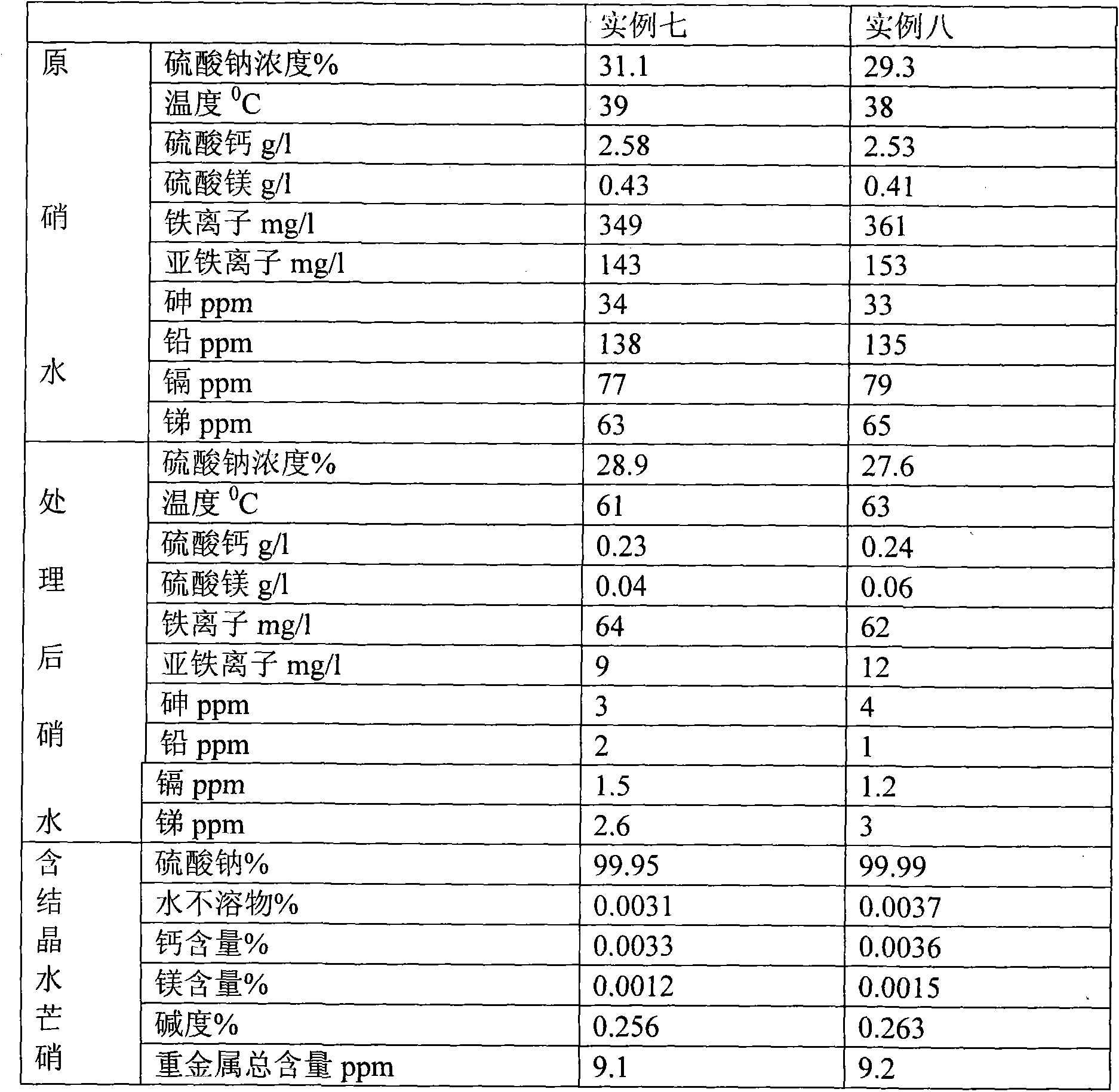
[0036]Use a pump to pump hot water at 40°C to dissolve the Glauber's salt (sodium sulfate) in the ore to form nitric water containing various impurities and a sodium sulfate concentration of 20.7%. Add 2641.4g / m 3 Sodium carbonate and 794.1g / m 3 Sodium sulfide, then add sodium hydroxide to make the pH value of nitric water reach 9; Impurities are removed, water is evaporated in a five-effect heat vacuum to form a saturated solution of Glauber’s salt and Glauber’s salt containing crystal water. After centrifugation, the Glauber’s salt and saturated solution containing crystal water are separated, and the saturated solution is returned to the five-effect heat vacuum evaporator for reuse. The thenardite containing crystal water is input into the hot-melt vacuum evaporation kettle to further remove the crystal water in the thenardite. When the moisture content in the dehydration kettle is reduced to 3%, the solid thenardite in the dehydration kettle is kept at 45°C for 0.5 hours. ...
Embodiment 2
[0038] Use a pump to pump hot water at 70°C to dissolve the Glauber's salt (sodium sulfate) in the ore to form nitric water containing various impurities and a sodium sulfate concentration of 32.1%. Add 2872.1g / m 3 Sodium carbonate and 836.1g / m 3 Sodium sulfide, then add sodium hydroxide to make the pH value of the nitric water reach 10; then add 0.15%wt polyaluminum ferric chloride sulfate (PAFCS) to settle the various precipitated substances generated, and finally add sulfuric acid to adjust the pH value of the nitric water Reach 7.1. In this way, after removing impurities and evaporating water in five-effect heat vacuum, a saturated solution of Glauber's salt and Glauber's salt containing crystal water is formed. After centrifugation, the Glauber's salt and saturated solution containing crystal water are separated, and the saturated solution is returned to five-effect heat for vacuum evaporation. The kettle is reused, and the thenardite containing crystal water is input int...
Embodiment 3
[0040] Use a pump to pump hot water at 50°C to dissolve the Glauber's salt in the ore to form nitric water containing various impurities and a sodium sulfate concentration of 25.8%. Add 2641.4g / m 3 Sodium carbonate and 889.2g / m 3 Sodium sulfide, then add sodium hydroxide to make the pH value of the nitric water reach 10; then add 0.25% wt polyaluminum ferric chloride sulfate (PAFCS) to settle the various precipitates generated, and finally add sulfuric acid to adjust the pH value of the nitric water Reach 7.4. In this way, after removing impurities and evaporating water in five-effect heat vacuum, a saturated solution of Glauber's salt and Glauber's salt containing crystal water is formed. After centrifugation, the Glauber's salt and saturated solution containing crystal water are separated, and the saturated solution is returned to five-effect heat for vacuum evaporation. The kettle is reused, and the thenardite containing crystal water is input into the hot-melt vacuum evapo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com