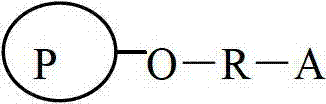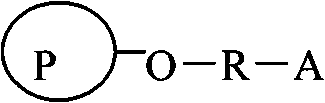Agarose compatible medium for purifying immune globulin of hand-foot-and-mouth disease and preparation method thereof
An immunoglobulin and hand, foot and mouth disease vaccine technology, applied in the biological field, can solve the problems of inability to treat hand, foot and mouth disease, low titer, limited separation accuracy, etc., and achieve excellent biocompatibility, high mechanical strength, high polymer The effect of exclusion limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Take 100.0 g of washed and drained agarose 6FF gel (GE healthcare company), pour it into a 1L three-necked flask, add 200 ml of 1,4-butanediol-diglycidyl ether, and 100 ml of 0.5M sodium hydroxide solution and 1.5g of sodium borohydride, stirred and reacted at 25°C for 10 hours, after the reaction was over, washed with water, ethanol, and water successively and vacuumed off the solvent to obtain epoxy-activated agarose gel; take 100g of epoxy-activated agar After the sugar gel was washed with water, drained, put into a 1L three-necked flask, added 100ml pH 8.0 hand, foot and mouth disease vaccine (Hualan Biological Co., Ltd.) solution, oscillated in a water bath at 37°C for 24 hours; 1mol / L ethanolamine was shaken and reacted at 37°C for 4 hours; the gel was sequentially washed with deionized water, 0.1M pH 4.0 acetic acid-sodium acetate buffer solution containing 0.5M NaCl, deionized water and 0.1M pH 8.0 boric acid containing 0.5M NaCl - Washing with sodium tetraborat...
Embodiment 2
[0045] Take 100.0 g of washed and drained agarose 6FF gel (GE healthcare company), pour it into a 1L three-necked flask, add 200 ml of 1,4-butanediol-diglycidyl ether, 100 ml of 4M sodium hydroxide solution and 1.5g of sodium borohydride, stirred and reacted at 60°C for 4 hours, after the reaction was completed, washed with water, ethanol, and water in turn and vacuumed off the solvent to obtain epoxy-activated agarose gel; take 100g of epoxy-activated agarose After washing the gel with water, drain it, put it into a 1L three-necked flask, add 200ml pH 14.0 hand, foot and mouth disease vaccine solution (Hualan Biological Co., Ltd.), shake it in a water bath at 37°C for 24 hours; filter it with suction, wash it with deionized water, and add 1mol / L ethanolamine at 37°C for 4 hours; the gel was sequentially washed with deionized water, 0.1M pH4.0 acetic acid-sodium acetate buffer containing 0.5M NaCl, deionized water and 0.1M pH8.0 containing 0.5M NaCl Washing with boric acid-so...
Embodiment 3
[0047] Take 100.0 g of washed and drained agarose 6FF gel (GE healthcare company), pour it into a 1L three-necked flask, add 200 ml of 1,4-butanediol-diglycidyl ether, and 100 ml of 0.5M sodium hydroxide solution and 1.5g of sodium borohydride, stirred and reacted at 60°C for 4 hours, after the reaction was over, washed with water, ethanol, and water successively and vacuumed off the solvent to obtain epoxy-activated agarose gel; take 100g of epoxy-activated agar After the sugar gel was washed with water, drained, put into a 1L three-necked flask, added 500ml pH 8.0 hand, foot and mouth disease vaccine solution (Hualan Biological Co., Ltd.), shaken in a water bath at 37°C for 24 hours; suction filtered, washed with deionized water, added 1mol / L ethanolamine was shaken and reacted at 37°C for 4 hours; the gel was sequentially washed with deionized water, 0.1M pH4.0 acetic acid-sodium acetate buffer containing 0.5M NaCl, deionized water and 0.1M pH8.0 containing 0.5M NaCl boric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com