Nerve growth factor sponginum and preparation method thereof
A nerve growth factor and sponge technology, applied in the field of medicine, can solve the problems of difficulty in achieving sustained release, cumbersome operation process, poor wound adhesion, etc., and achieves the effects of rich raw material sources, simple production process and reduced damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
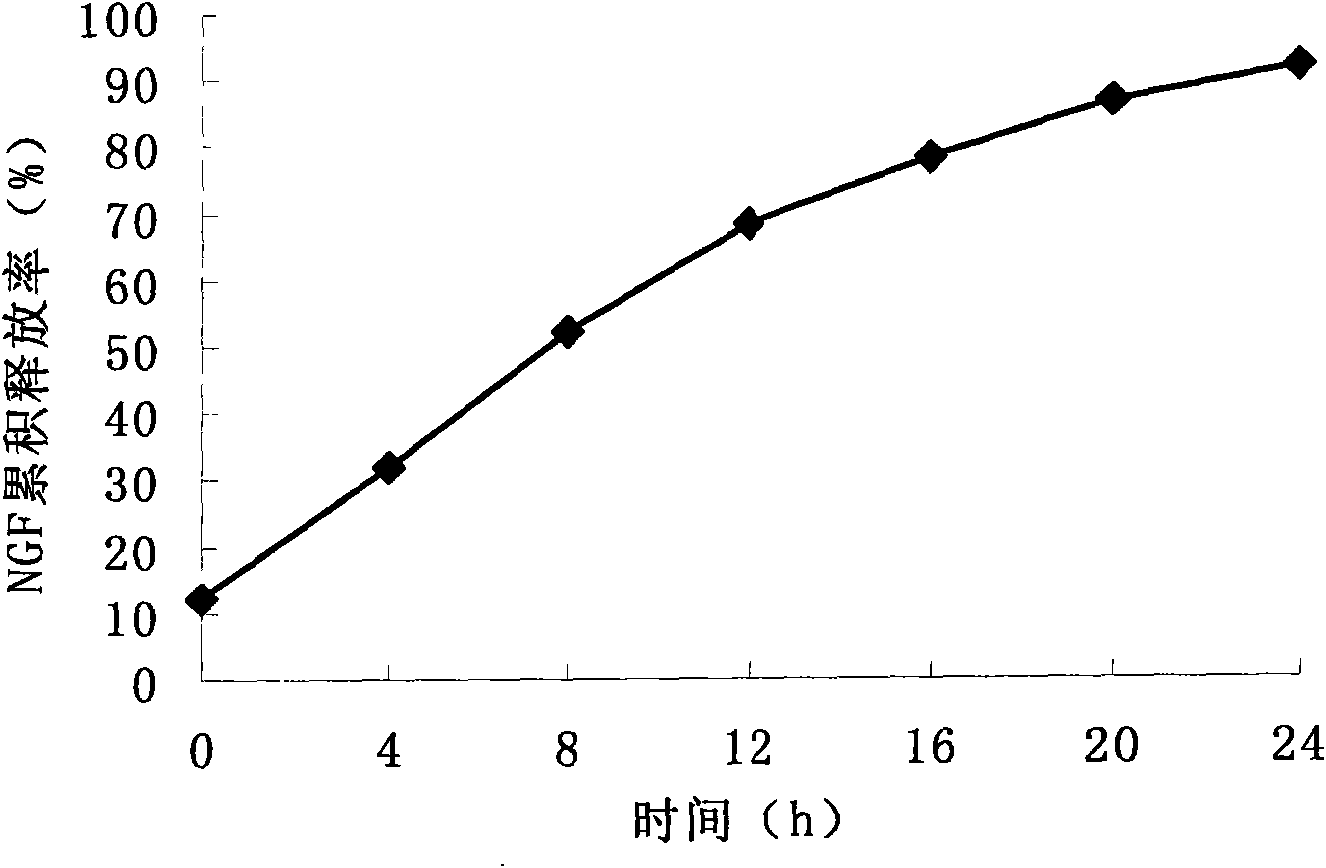
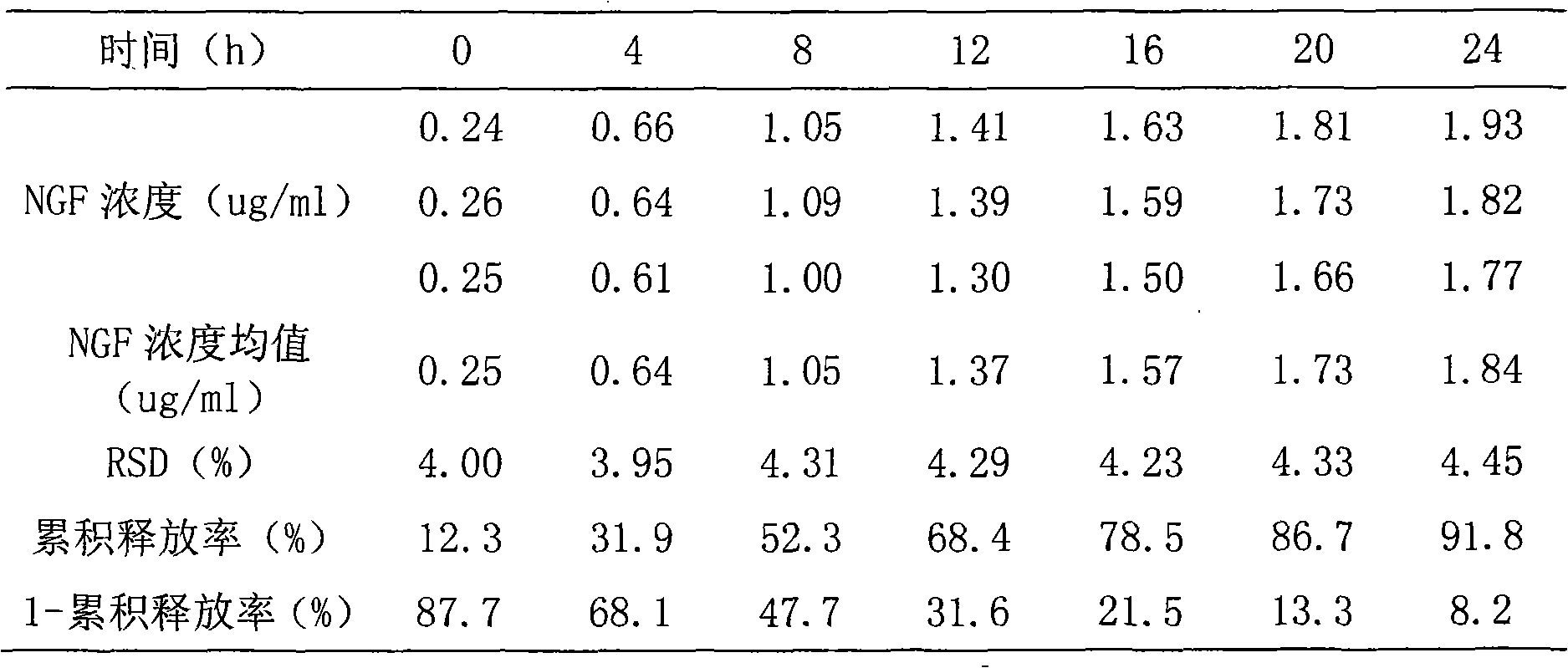
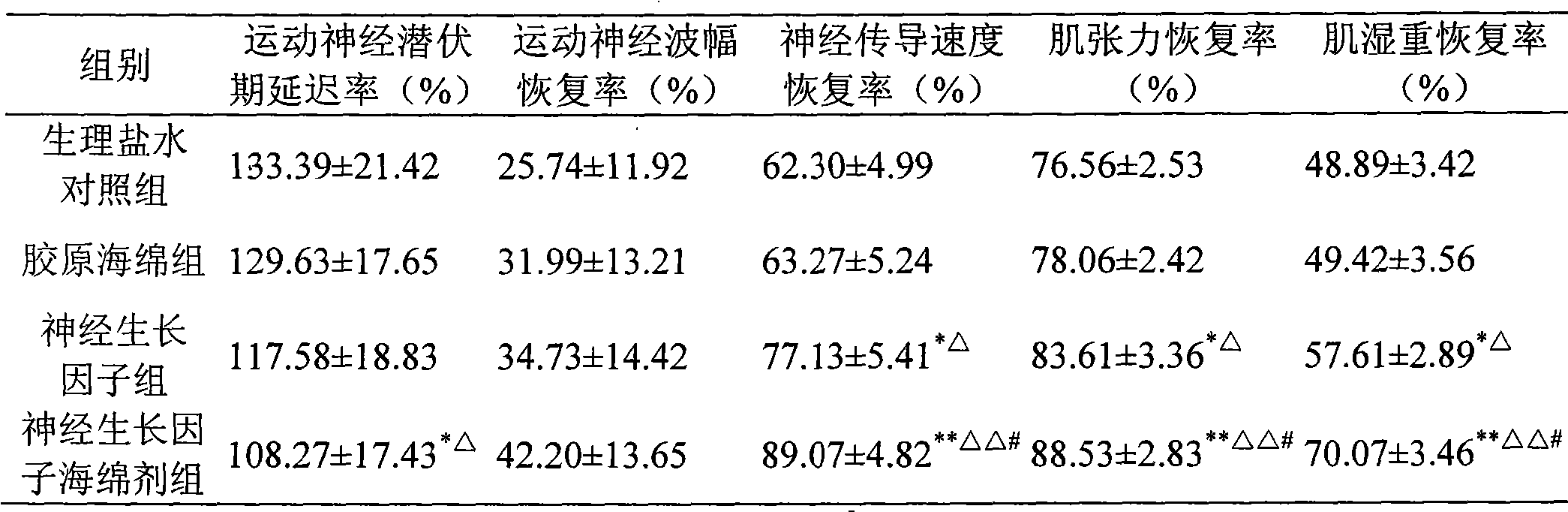
Image
Examples
Embodiment 1
[0047] Embodiment 1: Extraction and preparation of collagen
[0048] (1) Take the tendons of pork legs, remove impurities such as epidermis and fat, and clean them with cold purified water after slicing;
[0049] (2) Soak and wash with 20% sodium chloride for 3 times to degrease and remove impurities such as serum;
[0050] (3) Dissolve in 60 times the volume of 1% acetic acid solution, add pepsin at a final concentration of 0.4%, stir and digest at 37°C for 1 hour, then place it at 2-8°C for 5 days;
[0051] (4) Centrifuge the digestion solution, collect the supernatant, add NaCl to a final concentration of 2mol / L, precipitate overnight at 2-8°C, and collect the precipitate; redissolve the precipitate in 1% acetic acid solution, collect the supernatant by centrifugation, and add NaCl To a final concentration of 2 mol / L, precipitate overnight at 2-8°C, collect the precipitate, and obtain collagen.
Embodiment 2
[0052] Embodiment 2: Extraction and preparation of collagen
[0053] (1) Take the tendons of horse legs, remove impurities such as epidermis and fat, and clean them with cold purified water after slicing;
[0054] (2) Soak and wash with 20% sodium chloride twice to degrease and remove impurities such as serum;
[0055] (3) Dissolve in 30 times the volume of 1% acetic acid solution, add pepsin at a final concentration of 0.4%, stir and digest at 37°C for 1 hour, then place it at 2-8°C for 10 days;
[0056] (4) Centrifuge the digestion solution, collect the supernatant, add NaCl to a final concentration of 2mol / L, precipitate overnight at 2-8°C, and collect the precipitate; redissolve the precipitate in 0.1% acetic acid solution, collect the supernatant by centrifugation, and add NaCl To a final concentration of 1mol / L, precipitate overnight at 2-8°C, collect the precipitate, and obtain collagen.
Embodiment 3
[0057] Embodiment 3: Extraction and preparation of collagen
[0058] (1) Take the calf tendon, remove impurities such as epidermis and fat, and clean it with cold purified water after slicing;
[0059] (2) Soak and wash with 20% sodium chloride for 3 times to degrease and remove impurities such as serum;
[0060] (3) Dissolve in 50 times the volume of 1% acetic acid solution, add pepsin at a final concentration of 0.4%, stir and digest at 37°C for 1 hour, then place it at 2-8°C for 7 days;
[0061] (4) Centrifuge the digestion solution, collect the supernatant, add NaCl to a final concentration of 2mol / L, precipitate overnight at 2-8°C, and collect the precipitate; redissolve the precipitate in 0.5% acetic acid solution, collect the supernatant by centrifugation, and add NaCl To a final concentration of 1.5 mol / L, precipitate overnight at 2-8°C, collect the precipitate, and obtain collagen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com