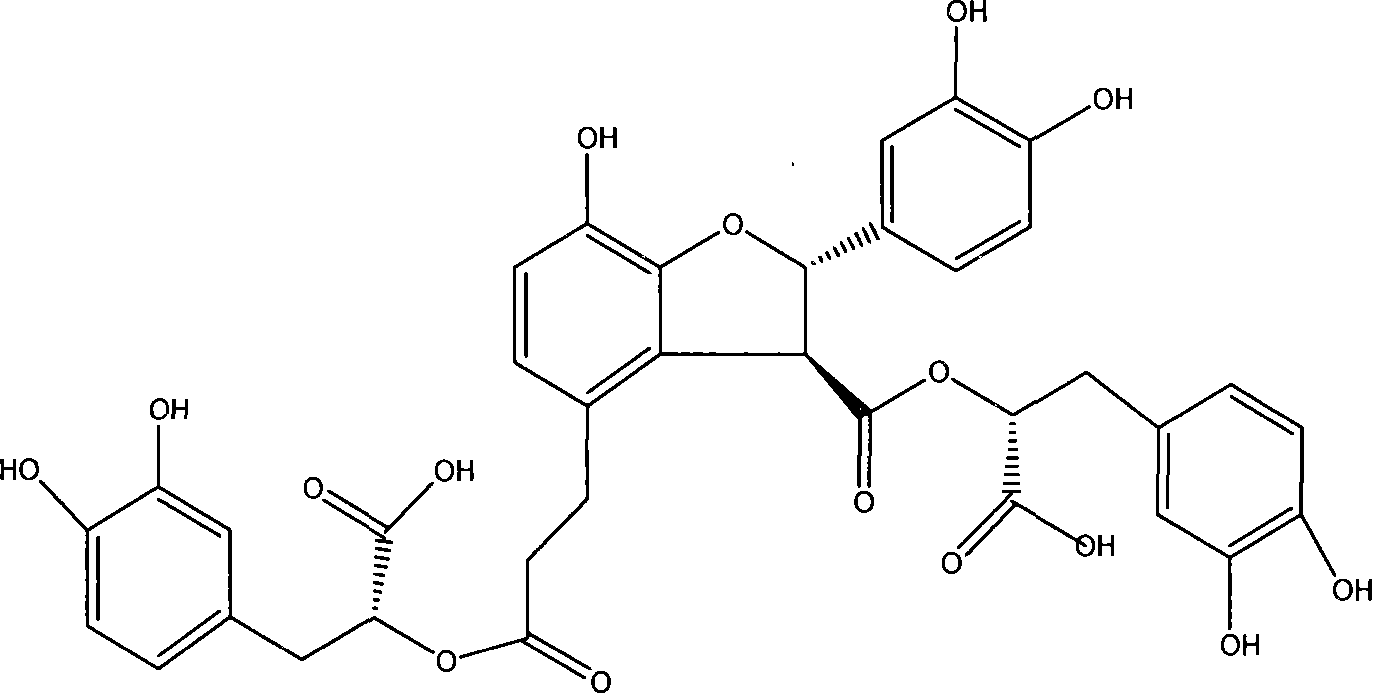
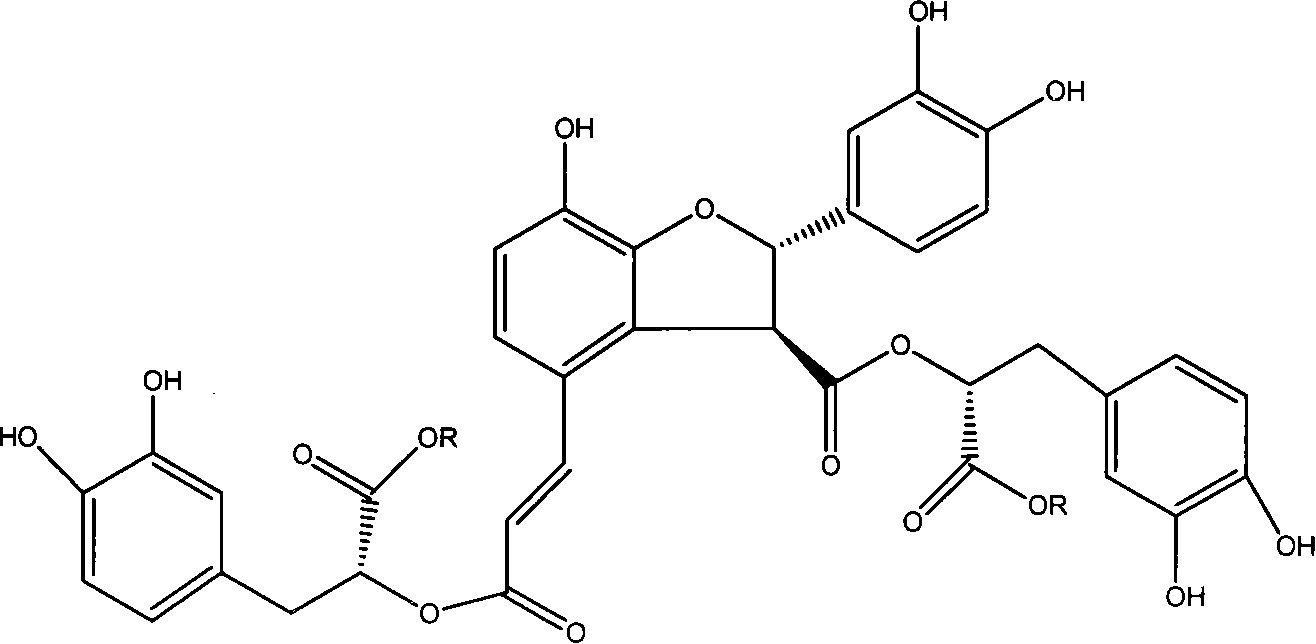
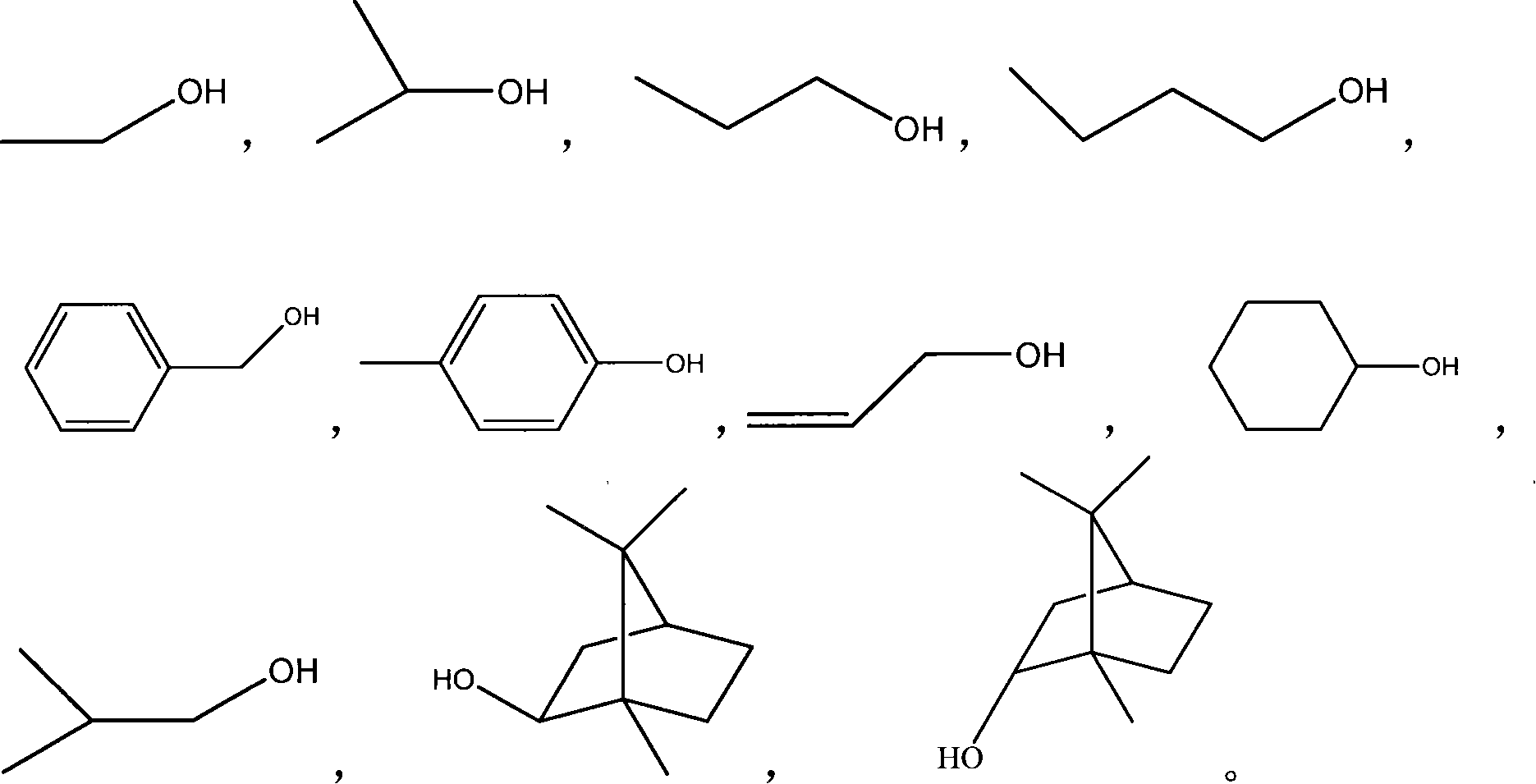
Application of structure modified products of salvianolic acid B in pharmacy
A technology of salvianolic acid and product, which is applied in the application field of the new compound, the structural modification product of salvianolic acid B in pharmaceuticals, can solve the problems of fast elimination, short half-life, difficulty, etc., to reduce the level of MDA and alleviate the disease. , Improve the effect of myocardial ischemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Protective effect of dihydrosalvianolic acid B on myocardial ischemia caused by coronary artery ligation
[0044] 50 Wistar rats, half male and half female, weighing 220-250 g, were randomly divided into three groups: the first group was the ischemic model group, the second group was the salvianolic acid B group, and the third group was the dihydrosalvianolic acid B group. In the group, the administration dose was 50 mg / kg intravenously; the electrocardiogram II was measured before the operation and 30 minutes after the ligation, and the ST segment and T wave changes of the electrocardiogram were observed.
[0045] In addition to the ischemia model group, the other two groups were dosed once 30 minutes after coronary artery ligation, and again after 4 hours, and blood was collected from the abdominal vein 24 hours later to measure serum LDH, CK, MDA, and SOD; Remove the rat heart, wash the remaining blood with normal saline, blot dry with filter paper, weigh, ...
Embodiment 2
[0058] Example 2 Effect of dihydrosalvianolic acid B on thrombus formation in rats
[0059] 30 Wistar rats, half male and half female, weighing 180-220g, were randomly divided into three groups: the first group was the blank control group, the second group was the salvianolic acid B group, and the third group was the dihydrosalvianolic acid B group , the dose of administration was intravenous injection of 50mg / kg; the first group was intravenously injected with the same volume of distilled water, and the other two groups were administered again after 3.5 hours of administration, a total of two administrations; anesthesia was performed 2 hours after the last administration , rats were fixed in the supine position, the common carotid artery was separated, and placed on the stimulating electrode and temperature probe hook of the BT87-3 experimental in vivo thrombosis analyzer. The time (s) required from the start of stimulation to the sudden drop of arterial blood vessel temperat...
Embodiment 3
[0065] Example 3 The protective effect of ethyl salvianolic acid B on myocardial ischemia caused by coronary artery ligation
[0066] Wistar rats, half male and half female, weighing 180-240g, were randomly divided into two groups: the first group was the ischemia model group, the second group was the salvianolic acid B ethyl ester group, and the dosage was 100 mg / kg by intragastric administration; Measure the lead II electrocardiogram before the operation and 30 minutes after the ligation, and observe the ST segment and T wave changes of the electrocardiogram.
[0067] After 30 minutes of coronary artery ligation, the dose was administered once, and again after 4 hours. After 24 hours, the heart of the rat was removed, the residual blood was washed with normal saline, dried with filter paper, weighed, and frozen. The ventricle was evenly cut into 5 parts, placed in 1% TTC solution (triphenyltetrazolium chloride) and stained for 15 minutes (temperature 37°C±0.5°C); after takin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com