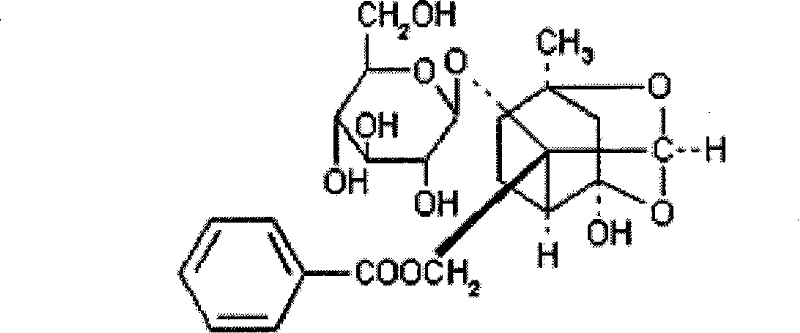
Preparation for percutaneous administration and preparation method and application thereof
A technology for drug delivery and preparation, applied in the field of transdermal drug delivery preparations and preparations containing paeoniflorin, can solve the problems of preparation stability, bioavailability difficult to meet requirements, unclear active ingredients, and difficulty in recovering viscosity, etc. The effect of lasting and stable drug concentration, improving bioavailability and convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 preferred paeoniflorin patch and preparation method thereof
[0047] Prescription: the following components are parts by weight
[0048] Paeoniflorin 1.5 parts
[0049] Carbomer (U10) 0.2 parts
[0050] Sodium polyacrylate (7S) 1.0 parts
[0051] Aluminum chloride 0.2 parts
[0052] Citric acid 0.015 parts
[0053] Glycerin 2.26 parts
[0054] Azone 0.5 parts
[0055] Propylene glycol 4 parts
[0056] 7 parts water
[0057] Ethylparaben 0.0021 parts
[0058] Preparation:
[0059] According to the prescription, ethylparaben is fully dissolved in distilled water and used as the water phase for later use; carbomer, paeoniflorin and appropriate amount of water phase are fully swollen and dispersed uniformly to obtain mixture I; sodium polyacrylate, glycerin, azone, propylene glycol and An appropriate amount of water was mixed uniformly to obtain mixture II; citric acid, aluminum chloride and the remaining water were mixed and dissolved to obtain mixtu...
Embodiment 2
[0062] Preferred paeoniflorin patch of embodiment 2 and preparation method thereof
[0063] Prescription: the following components are parts by weight
[0064] Paeoniflorin 1.0 part
[0065] Carbomer (980) 0.25 parts
[0066] Sodium polyacrylate (7M) 0.8 parts
[0067] Aluminum hydroxide 0.018 parts
[0068] Tartaric acid 0.02 parts
[0069] Glycerin 2.5 parts
[0070] Azone 0.5 parts
[0071] 1 part propylene glycol
[0072] 7.5 parts of water
[0073] Methylparaben 0.002 parts
[0074] Preparation:
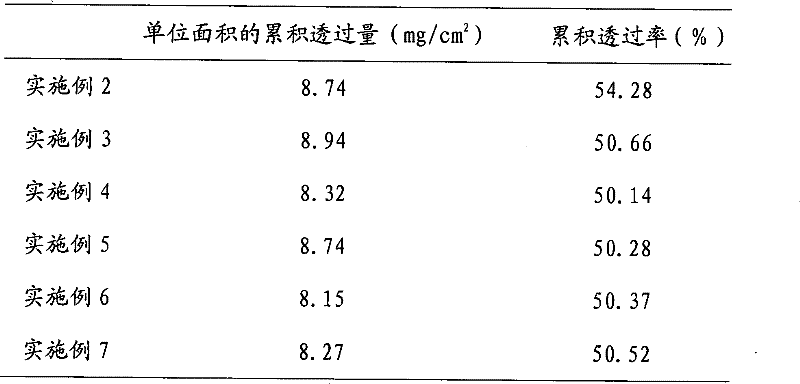
[0075] Take methylparaben according to the prescription and fully dissolve it in distilled water for later use; prepare according to the preparation method of Example 1, and obtain paeoniflorin-containing 15.7mg / cm 2 The patch has a water content of about 8.9%, just pack it.
Embodiment 3
[0076] Embodiment 3 preferred paeoniflorin patch and preparation method thereof
[0077] prescription:
[0078] Paeoniflorin 0.8 parts
[0079] 0.1 parts of polyvinyl alcohol
[0080] Cross-linked sodium polyacrylate (NP-700) 0.8 parts
[0081] Magnesium hydroxide 0.025 parts
[0082] Tartaric acid 0.02 parts
[0083] Sorbitol 2.8 parts
[0084] 0.7 parts of 1-methyl-2-pyrrolidine
[0085] 6.8 parts of water
[0086] Preparation:
[0087] Prepared according to the preparation method of Example 1, the difference is that there is no preservative, that is, 12.5 mg / cm of paeoniflorin is obtained. 2 The patch can be packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com