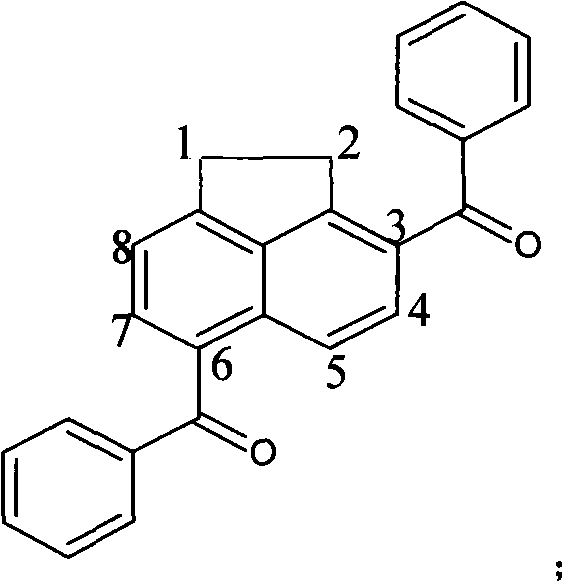
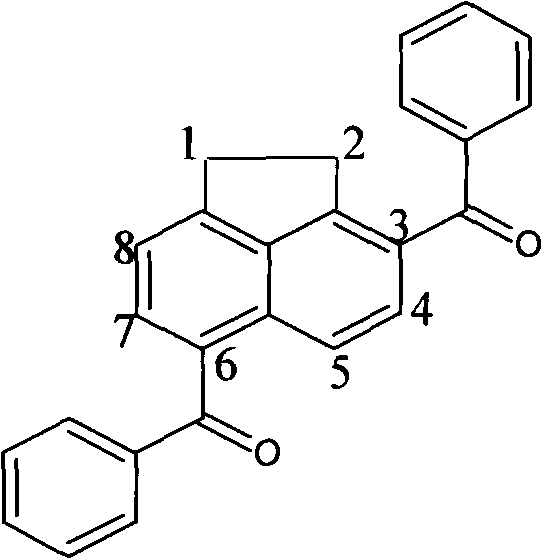
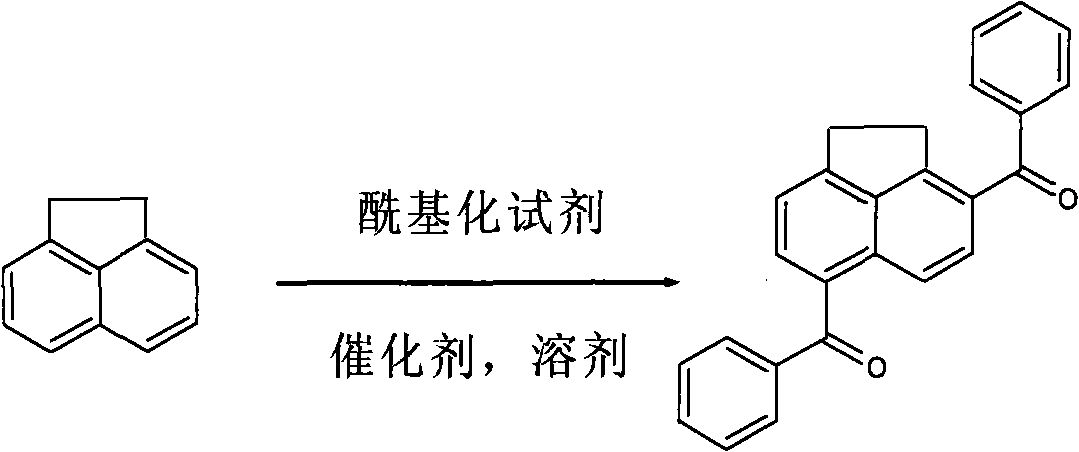
Polycyclic arone 3,6-diphenyl acenaphthene and synthesis method thereof
A technology of dibenzoyl acenaphthene and polycyclic aromatic ketone, which is applied in the field of polycyclic aromatic ketone 3,6-dibenzoyl acenaphthylene and its synthesis, can solve the problems of only 33% yield and low yield, and achieve The product synthesis process is simple, the reaction is simple, and the selectivity is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment one: with CCl 4 as solvent, anhydrous AlCl 3 as catalyst, benzoyl chloride as acylating reagent
[0032] 1.08gAlCl 3 (8mmol, treated by sublimation) was added to 10ml of CCl 4 In a 100ml three-necked flask with a spherical reflux condenser, 2.0ml benzoyl chloride (8mmol) was added dropwise under magnetic stirring, and stirred until AlCl 3Dissolve and mix; then dissolve 0.154g acenaphthene (1mmol) in 5ml CCl 4 , and slowly drop it into a three-necked flask, then place the three-necked flask in a 45°C constant temperature water bath, turn on magnetic stirring, stop stirring after 10 hours of reaction, take out the flask, and let the reaction system drop to room temperature; the reaction mixture Pour it into distilled water, stir well, pour it into a separatory funnel and let it stand for stratification, take out the organic phase, extract the water phase with chloroform for 3 to 4 times, combine the extract and the organic phase with deionized water, satura...
Embodiment 2
[0033] Embodiment two: with CCl 4 as solvent, ionic liquid [Emim]Cl-AlCl 3 as catalyst, benzoyl chloride as acylating reagent
[0034] First, the ionic liquid [Emim]Cl-AlCl must be specially prepared 3 (See literature: Chen Min, Chemical Reagent, 2007, 29(10): 628). Methods as below:
[0035] ①Synthesis of ionic liquid intermediate [Emim]Cl: in N 2 Under the atmosphere, take equimolar N-methylimidazole and ethyl chloride dried by phosphorus pentoxide, and react under reflux at 80-82°C for 24h, cool down, and stand to separate layers. Freeze at -30°C for 12 hours, pour out the upper layer liquid, add 15ml of acetonitrile, stir and dissolve at 80°C, then add 250ml ethyl acetate, stir, freeze at -30°C for 12h, pour out the upper layer liquid, and put the crude product at 80°C Rotary evaporation for 10 h gave 1-ethyl-3-methylimidazole chloride ([Emim]Cl).
[0036] ②Ionic liquid [Emim]Cl-AlCl 3 Synthesis: Weigh [Emim]Cl and AlCl respectively according to different molar rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com