Human epididymis protease profilin immunological contraception polypeptide
A protease inhibition, immune contraceptive technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
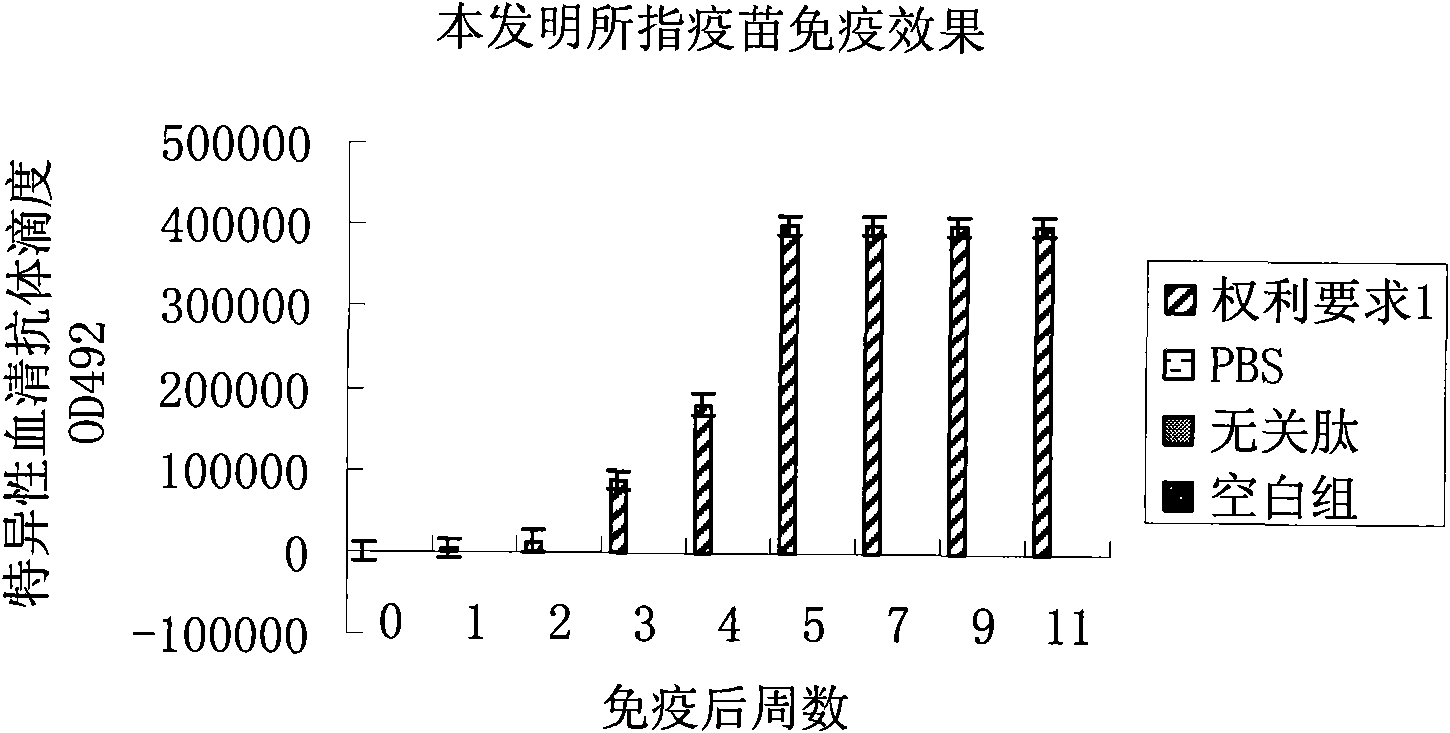
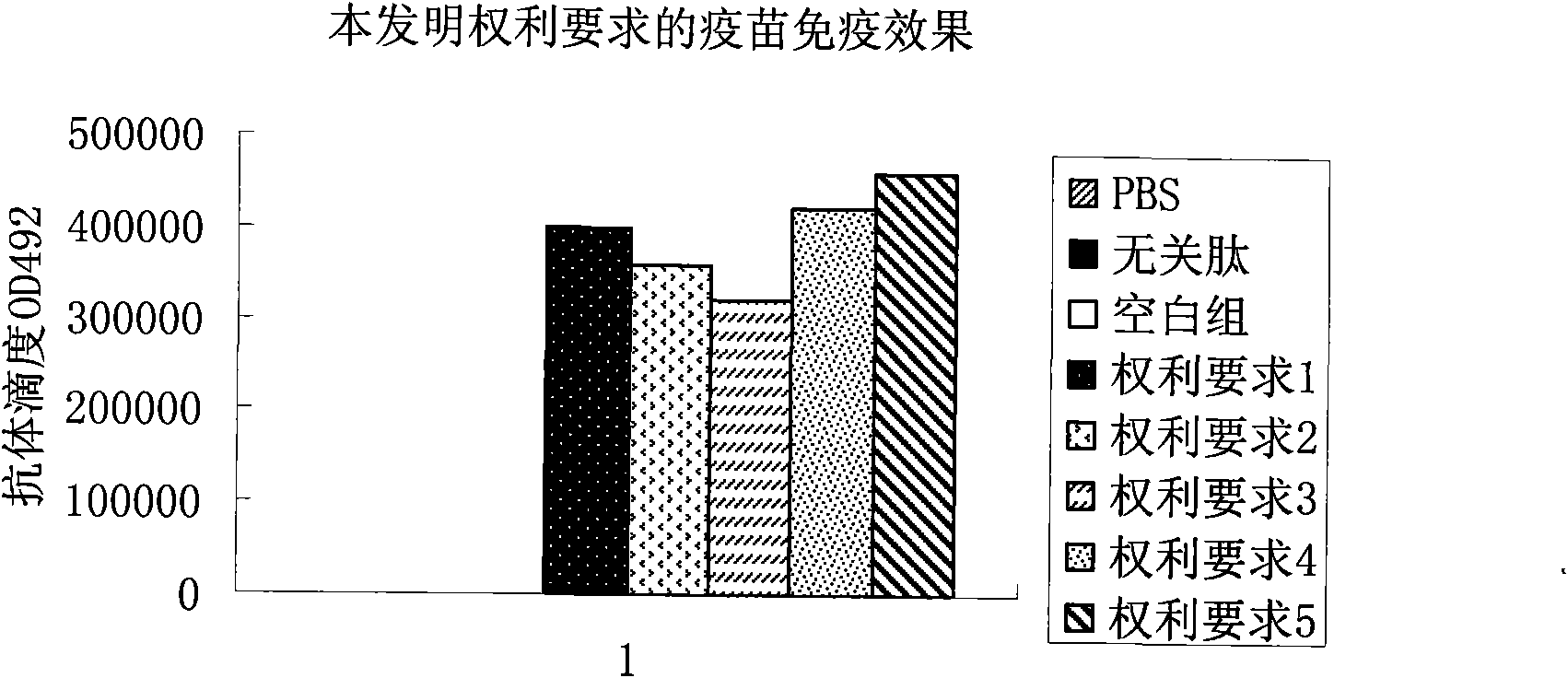
[0055] Example 1: The basic amino acid sequence of the present invention is: amino-terminal-LSEIKGVIVHRLEGVGGGMFVYGGCQGNNNN-carboxyl-terminal; using lysine as a linker, multiple basic amino acid sequences form a multi-copy tandem structure.
[0056] The use of the basic amino acid sequence referred to in the present invention:
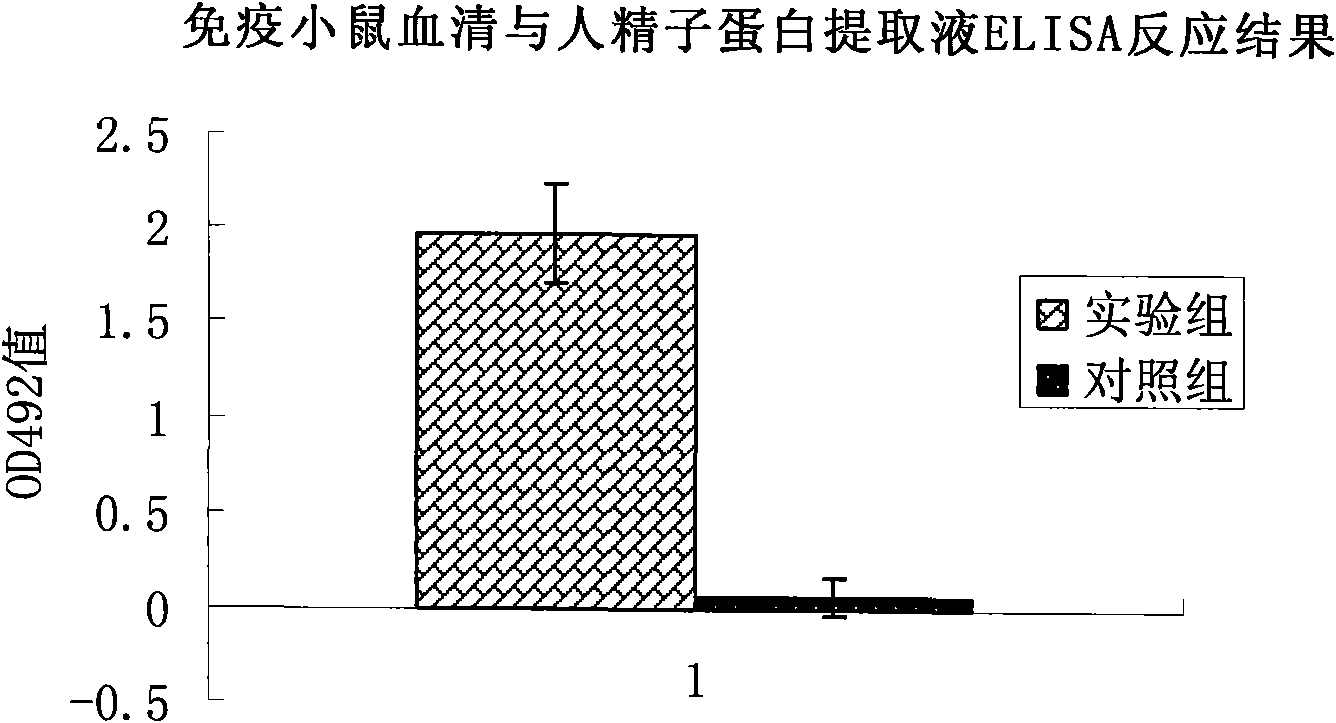
[0057] Mix with Freund's adjuvant in equal amount at a dose of 80 micrograms / mouse / time, and inject into the subcutaneous tissue of the groin of Balb / c mice; 7 days later, inject Balb / c at a dose of 50 micrograms / mouse / time in the same way The subcutaneous tissue site of the groin of the mouse; after another 14 days, inject the subcutaneous tissue site of the groin of the Balb / c mouse at a dose of 50 μg / time / only in the same way. After 28 days of observation, the tail veins of the immunized mice were bled, and their serum was collected. The antibody titer in the mouse serum was detected by enzyme-linked immunosorbent assay (ELISA), which was significan...
Embodiment 2
[0090] Embodiment 2: The basic amino acid sequence of the present invention can also be:
[0091]
[0092] Main reagents and their preparation:
[0093] Coating diluent (0.05mol / L sodium carbonate-sodium bicarbonate buffer, pU9.6) composition: Na 2 CO 3 1.5g, NaHCO 3 2.9g, Na 2 N 3 0.2g, add double distilled water to 1000ml, adjust to pH9.6.
[0094] Blocking solution (5% calf serum / PBS solution) composition:
[0095] Calf serum 50ml, add PBS (pH7.4) 950ml.
[0096] Phosphate buffered saline (PBS):
[0097] A liquid (0.2mol / L sodium dihydrogen phosphate aqueous solution) composition: NaH 2 PO 4 ·H 2 27.6g of O was dissolved in 1000ml of ultrapure water.
[0098] B solution (0.2mol / L disodium hydrogen phosphate aqueous solution) composition: Na 2 HPO 4 ·7H 2 O 53.6g (or Na 2 HPO 4 12H 2 O71.6g or Na 2 HPO 4 2H 2 O 35.6g) was dissolved in 1000ml of ultrapure water.
[0099] Sample diluent (PBS, 0.01mol / L phosphate-buffered saline) composition: PBA solu...
Embodiment 3
[0122] Embodiment 3: The basic amino acid sequence of the present invention can also be:
[0123]
[0124] Main reagents and their preparation:
[0125] Coating diluent (0.05mol / L sodium carbonate-sodium bicarbonate buffer, pU9.6) composition: Na 2 CO 3 1.5g, NaHCO 3 2.9g, Na 2 N 3 0.2g, add double distilled water to 1000ml, adjust to pH9.6.
[0126] Blocking solution (5% calf serum / PBS solution) composition:
[0127] Calf serum 50ml, add PBS (pH7.4) 950ml.
[0128] Phosphate buffered saline (PB):
[0129] A liquid (0.2mol / L sodium dihydrogen phosphate aqueous solution) composition: NaH 2 PO 4 ·H 2 27.6g of O was dissolved in 1000ml of ultrapure water.
[0130] B solution (0.2mol / L disodium hydrogen phosphate aqueous solution) composition: Na 2 HPO 4 ·7H 2 O 53.6g (or Na 2 HPO 4 12H 2 O71.6g or Na 2 HPO 4 2H 2 O 35.6g) was dissolved in 1000ml of ultrapure water.
[0131] Sample diluent (PBS, 0.01mol / L phosphate-buffered saline) components: PBA soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com