Waterborne polyurethane and synthetic method thereof
A technology of water-based polyurethane and synthesis method, which is applied in the field of polymer chemistry and polymer material science, can solve the problems of low content, consumption, and low molecular weight of the polymer, and achieve the effect of simple synthesis method and easy realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
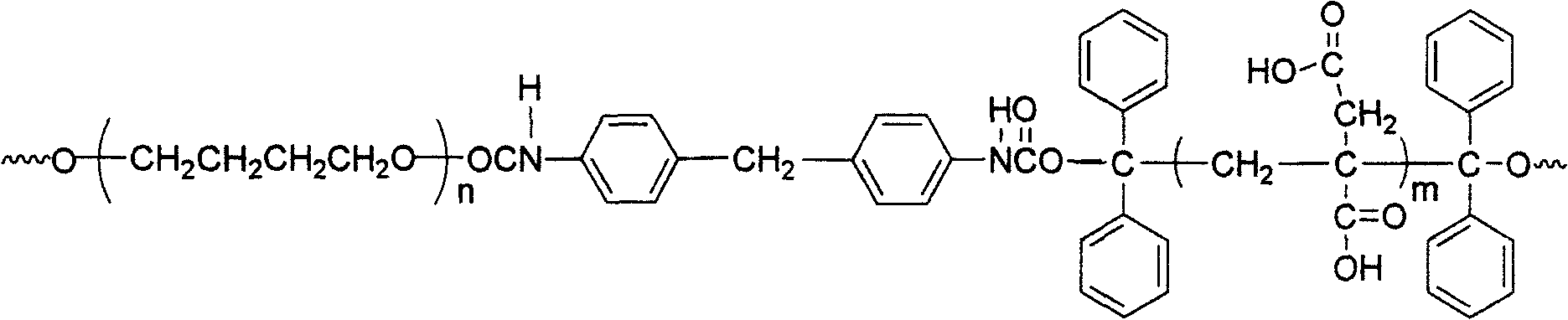
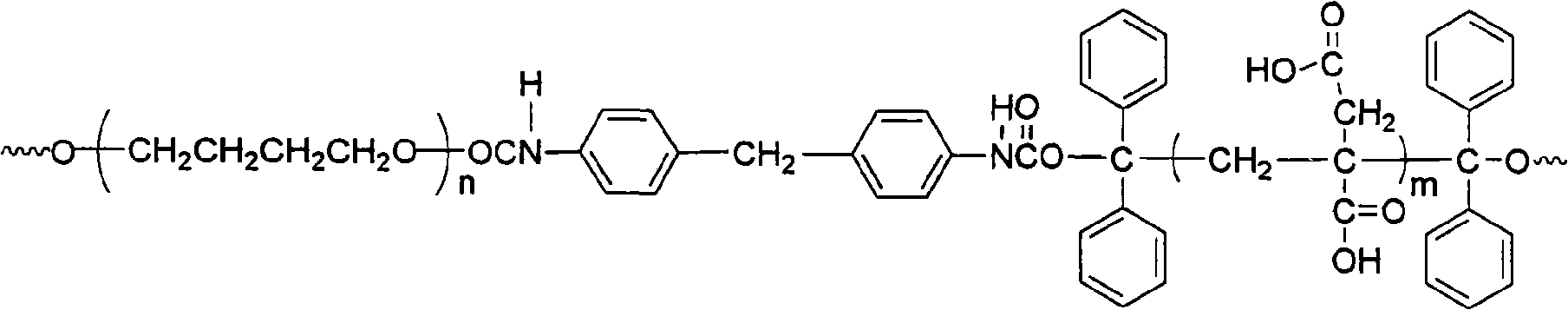
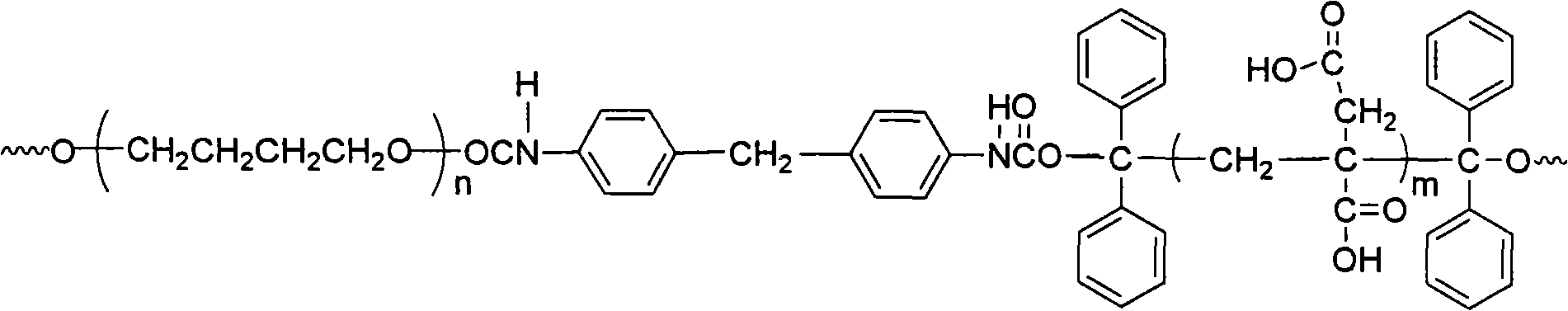
[0022] The method for synthesizing water-based polyurethane proposed by the present invention uses raw materials including benzophenone I, isopropanol II, polymer glycol III, diisocyanate IV, itaconic acid V and vinylpyrrolidone VI, and the structure of each raw material The expression is:
[0023]
[0024] The synthesis method includes the following steps:
[0025] (1) Mix 0.05-10 mol of benzophenone and 0.25-50 mol of isopropanol, and then add 0.01 to 0.2% of the first catalyst, which accounts for the total mass of benzophenone and isopropanol, at a temperature of 10 to 35°C. At temperature, react under ultraviolet light for 1-10 hours to obtain 1,1,2,2-tetraphenylethylene glycol. The reaction formula is as follows:
[0026]
[0027] (2) Mix 0.01-10 mol polymer diol and 0.03-20 mol diisocyanate, add solvent, stir and mix at 60-85°C under the protection of nitrogen for 1 to 4 hours to obtain a prepolymer; the temperature is reduced to 20-45 ℃, then add the above 1,1,2,2-tetrapheny...
Embodiment 1
[0033] (1): First mix benzophenone (0.1mol) and isopropanol (0.5mol), then add acetic acid (0.005mol), the mixture is charged into a 500mL round-bottomed flask, at a temperature of 25℃, After exposure to 365nm ultraviolet light, the product (1,1,2,2-tetraphenylethylene glycol) was precipitated, and the precipitated product was purified by recrystallization from acetic acid.
[0034] The product was characterized by NMR, 1 H-NMR(CDCl 3 ): δ=3.05 (0H, 2H), 7.00-7.50 (phenyl, 20H).
[0035] (2): Mix polytetrahydrofuran ether glycol (0.01mol) and 4,4'-diphenylmethane diisocyanate (0.02mol) into 100mL tetrahydrofuran, and the mixture is charged into a 500mL three-neck round bottom flask, protected by nitrogen Stir and mix and react for 3 hours at 75°C to obtain a prepolymer; the temperature is reduced to 35°C, and 1,1,2,2-tetraphenylethylene glycol (0.01mol) is added, and the molar ratio of the addition is: 1,1 , 2,2-tetraphenylethylene glycol: diisocyanate = 1:2, then add 0.02% dibuty...
Embodiment 2
[0040] (1): First mix benzophenone (0.1mol) and 2-propanol (0.5mol), and then add acetic acid (0.005mol). The mixture is put into a 500mL round-bottomed flask and kept at a temperature of 30℃ After exposure to 365nm ultraviolet light, the product (1,1,2,2-tetraphenylethylene glycol) was precipitated, and the precipitated product was purified by recrystallization from acetic acid.
[0041] (2): Mix polycarbonate 1,4-butanediol ester diol (0.01mol) and 2,6-toluene diisocyanate (0.02mol) into 100mL methyl ethyl ketone, and the mixture is charged into a 500mL three-necked round bottom flask , Under the protection of nitrogen, stir and mix and react at 85°C for 3 hours to obtain a prepolymer; the temperature is reduced to 35°C, and 1,1,2,2-tetraphenylethylene glycol (0.01mol) is added, and the molar ratio is :1,1,2,2-tetraphenylethylene glycol: diisocyanate = 1:2, then add 0.03% of stannous octoate based on the total weight of the reactants, stir and react for about 10 hours; the prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com