Tumor targeting recombinant DNA vaccine, preparation method thereof and application thereof
A DNA vaccine and tumor-targeting technology, which is applied in the field of tumor-targeted recombinant DNA vaccines and DNA vaccines, to achieve the effects of fewer vaccinations, easy transportation and use, and low preparation costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Cloning of COX-2 gene
[0043] Extraction of total mRNA from A549 cells
[0044] A549 cells were cultured in complete 1640 medium containing 10% calf serum at 37°C and 5% CO 2 After trypsinization, the cells were centrifuged to collect the cells in an incubator, and the supernatant was discarded. The total mRNA was extracted according to the Trizol extraction kit (Hua Shun Company), and detected by electrophoresis.
[0045] Primer design and RT-PCR amplification
[0046] Primers were designed according to the COX-2 gene sequence provided by the accession number BC013734 of the GenBank database and the restriction sites on the pVAX1 vector, and BamH I and EcoR I restriction sites were introduced into the upper and lower primers respectively. The primers were provided by Shanghai Saibaisheng Gene Technology Co., Ltd. synthesis:
[0047] Upstream primer (SEQ ID NO 8): 5'-CG GGATCC GGTGGGGGCGGTAACGTTCCTTGCTGTTCCAACCCATGTC-3` (the BamH I site is underlined, pa...
Embodiment 2
[0078] Example 2 Cloning of murine mUbi gene
[0079] Extraction of total mRNA from mouse testis tissue
[0080] The testicular tissues of C57BL / 6 male mice (Shanghai Experimental Animal Center, Chinese Academy of Sciences) were washed under sterile conditions with DEPC-treated double distilled water twice, and 0.5 g of tissues were taken, crushed, and extracted according to the Trizol Kit (Hua Shun Company) The total mRNA was extracted and detected by electrophoresis.
[0081] Primer design and RT-PCR amplification
[0082] Primers were designed according to the mUbi gene sequence provided by the GenBank database X51703 accession number and the restriction site on the pVAX1 vector, and the HindIII and BamH I restriction sites were respectively introduced into the upper and lower primers. The primers were synthesized by Shanghai Saibaisheng Gene Technology Co., Ltd.:
[0083] Upstream primer (SEQ ID NO 10): 5'-CG AAGCTT GCCACCATGCAGATCTTCGTGAAAACC-3` (the underline is the ...
Embodiment 3
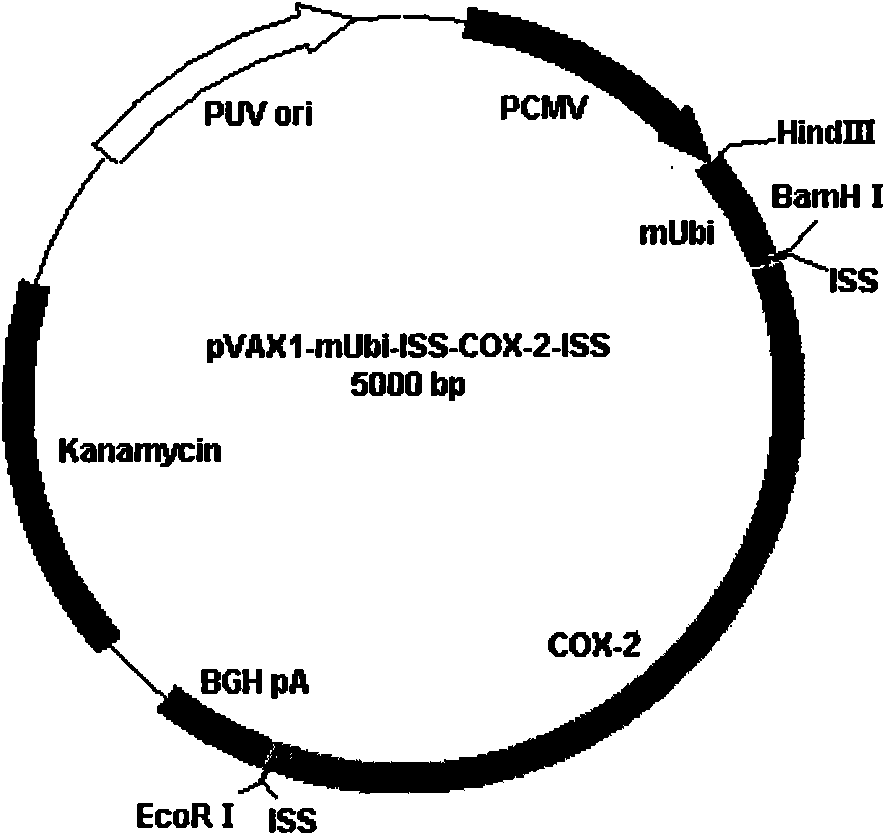
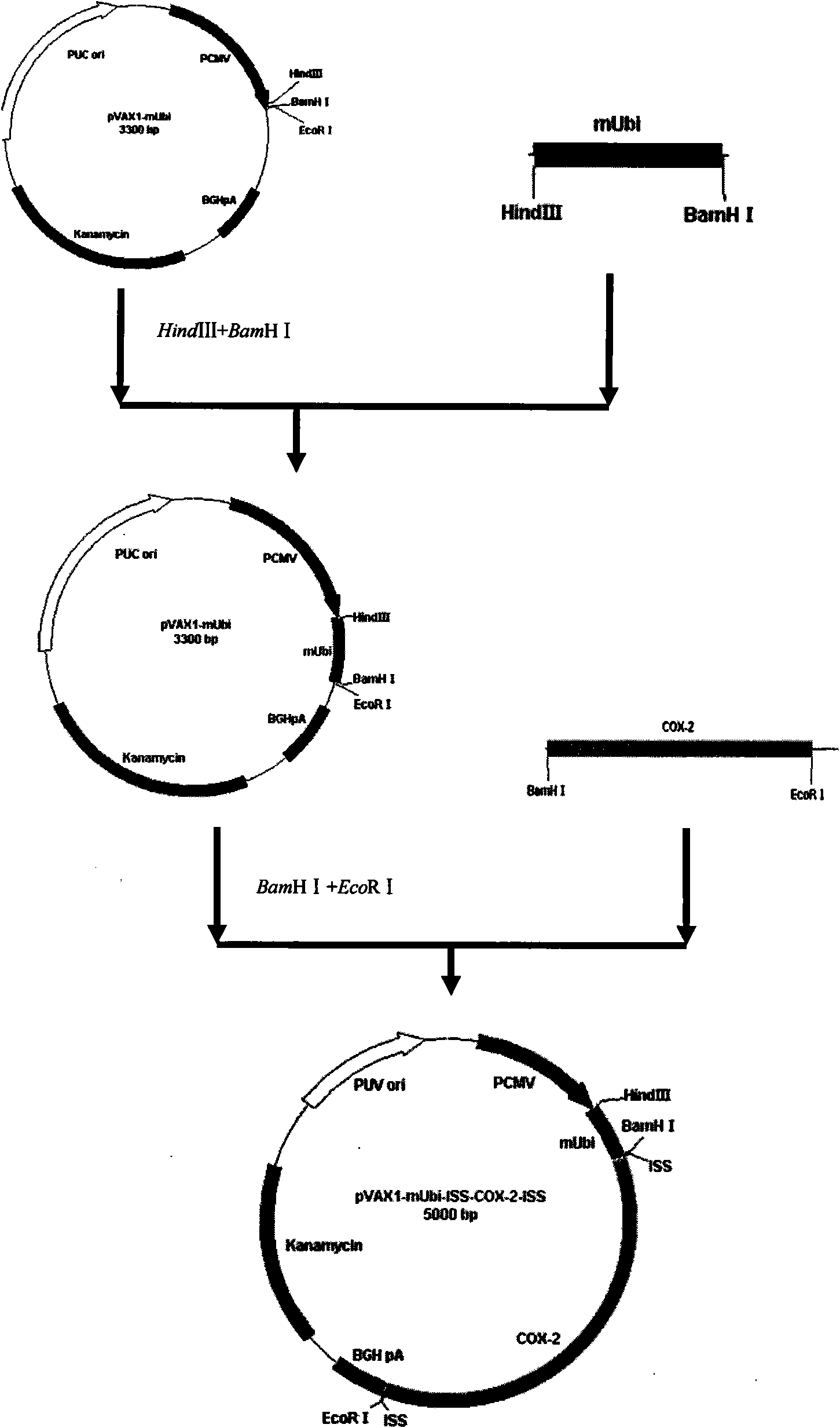
[0105] Example 3 Construction of pVAX1-mUbi-ISS-COX-2-ISS Tumor Targeting DNA Vaccine Recombinant Vector
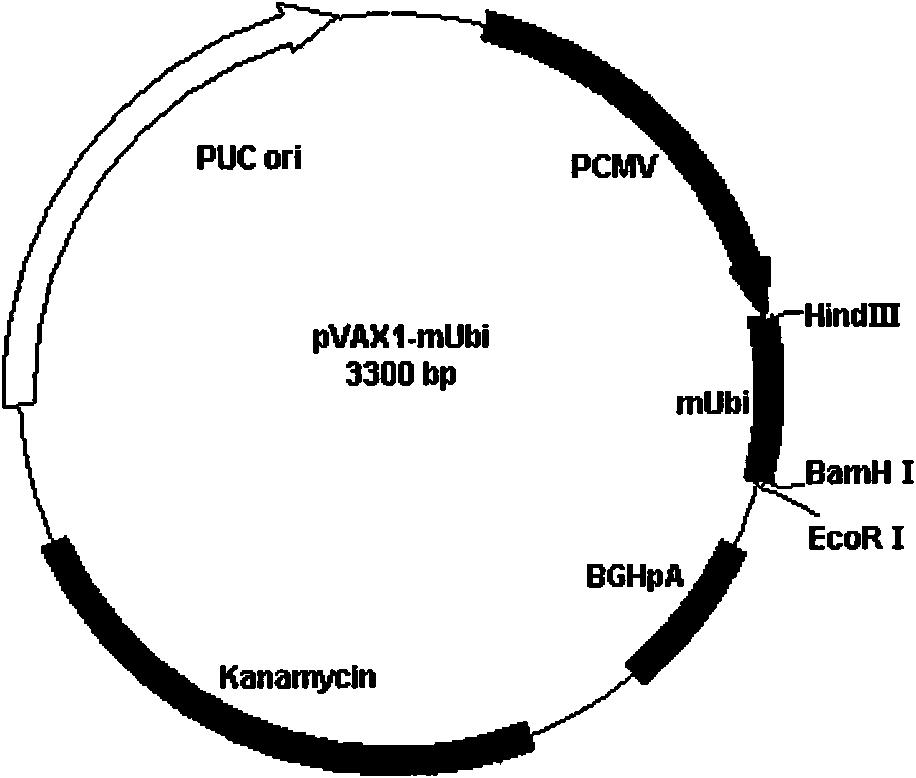
[0106] Construction of pVAX1-mUbi recombinant vector
[0107] Incubate 5 mL of LB containing 20 μg / mL Amp resistance, and culture DH5a-competent Escherichia coli containing pMD19-mUbi plasmid overnight at 37°C with shaking, and extract pMD19-mUbi plasmid DNA according to the third edition of "Molecular Cloning Experiment Guide".
[0108] Also culture 5 mL of LB containing 50 μg / mL Kan resistance, and culture DH5a-competent Escherichia coli containing pVAX1 plasmid overnight at 37°C with shaking, and extract pVAX1 plasmid DNA according to the third edition of "Molecular Cloning Experiment Guide".
[0109] Digest pMD19-mUbi plasmid DNA and pVAX1 plasmid DNA (Invitrogen company) with HindIII and BamH I (TaKaRa company), respectively, and the digestion reaction system is 80 μL:
[0110] Plasmid DNA 20μL
[0111] HindIII 3μL
[0112] BamH I 3 μL
[0113] 10×buffer K 8μL
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com