Dicyclohexyl trifluoromethanesulfonate ammonium salt and application thereof
A technology of ammonium dicyclohexyltrifluoromethanesulfonate and trifluoromethanesulfonic acid, which is applied in the direction of sulfonate preparation, carboxylic acid amide preparation, formation/introduction of amide groups, etc., and can solve the problem of long reaction time and substrate Low applicability, difficult catalyst recovery, etc., to achieve the effect of low cost, recyclable price, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Under ice-bath conditions, trifluoromethanesulfonic acid (0.750g, 5mmol) was slowly added dropwise to a toluene solution (10mL) of dicyclohexylamine (0.905g, 5mmol), and the reaction temperature was controlled at -5°C to 5°C The mixture was reacted for 30 minutes, washed with dichloromethane and concentrated to obtain 1.40 g of dicyclohexylamine ammonium trifluoromethanesulfonate, with a yield of 85%.
Embodiment 2
[0037] Under ice-bath conditions, trifluoromethanesulfonic acid (0.750g, 5mmol) was slowly added dropwise to a cyclohexane solution (10mL) of dicyclohexylamine (0.905g, 5mmol), and the reaction temperature was controlled at -5°C to React at 5°C for 30 minutes, wash with water and concentrate to obtain 1.42 g of ammonium dicyclohexylamine trifluoromethanesulfonate, with a yield of 86%.
Embodiment 3
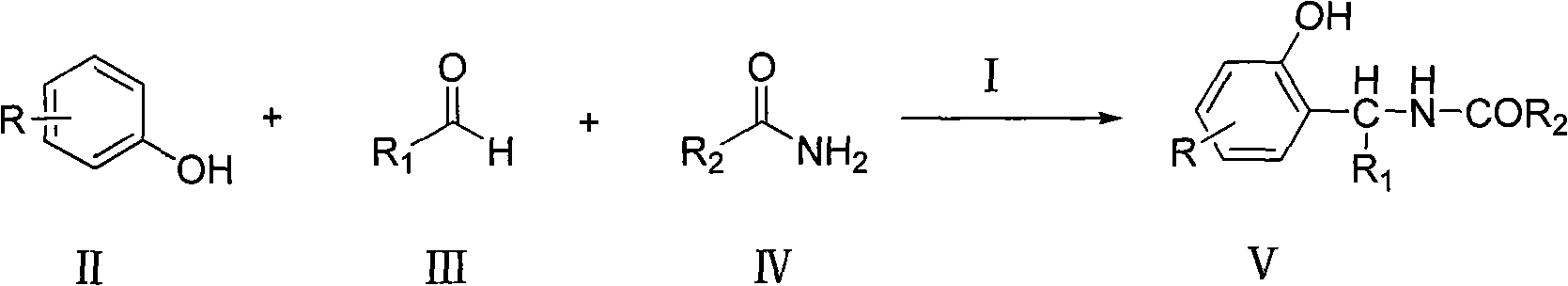
[0039] Add 2-naphthol (0.144g, 1.0mmol), benzaldehyde (0.106g, 1.0mmol), benzamide (0.133g, 1.1mmol) and ammonium trifluoromethanesulfonate in a 25mL two-necked flask (0.033g, 0.1mmol), reacted under the reflux condition of chloroform (2mL), followed by TCL. After the reaction, extract with water and ethyl acetate, take the organic layer, concentrate, and recrystallize from ethyl acetate to obtain the corresponding condensation product with a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com