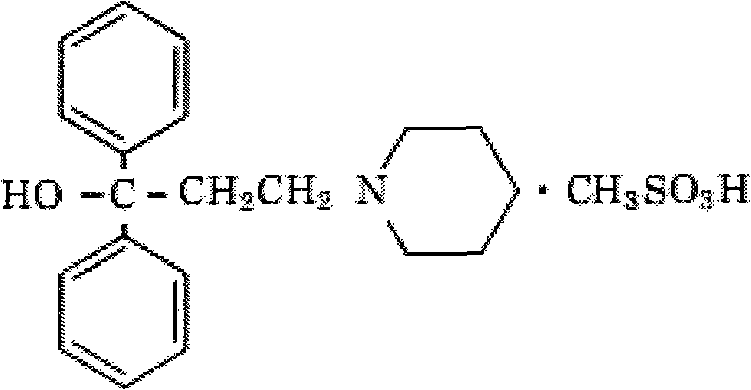
Methylsulfonic acid pridinol oral disintegrating tablet and preparation method thereof
A technology of orally disintegrating tablets and pridinol, which is applied to the orally disintegrating tablets of pridinol mesylate. Report and other issues, to achieve the effect of good medication compliance, simple production process, and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
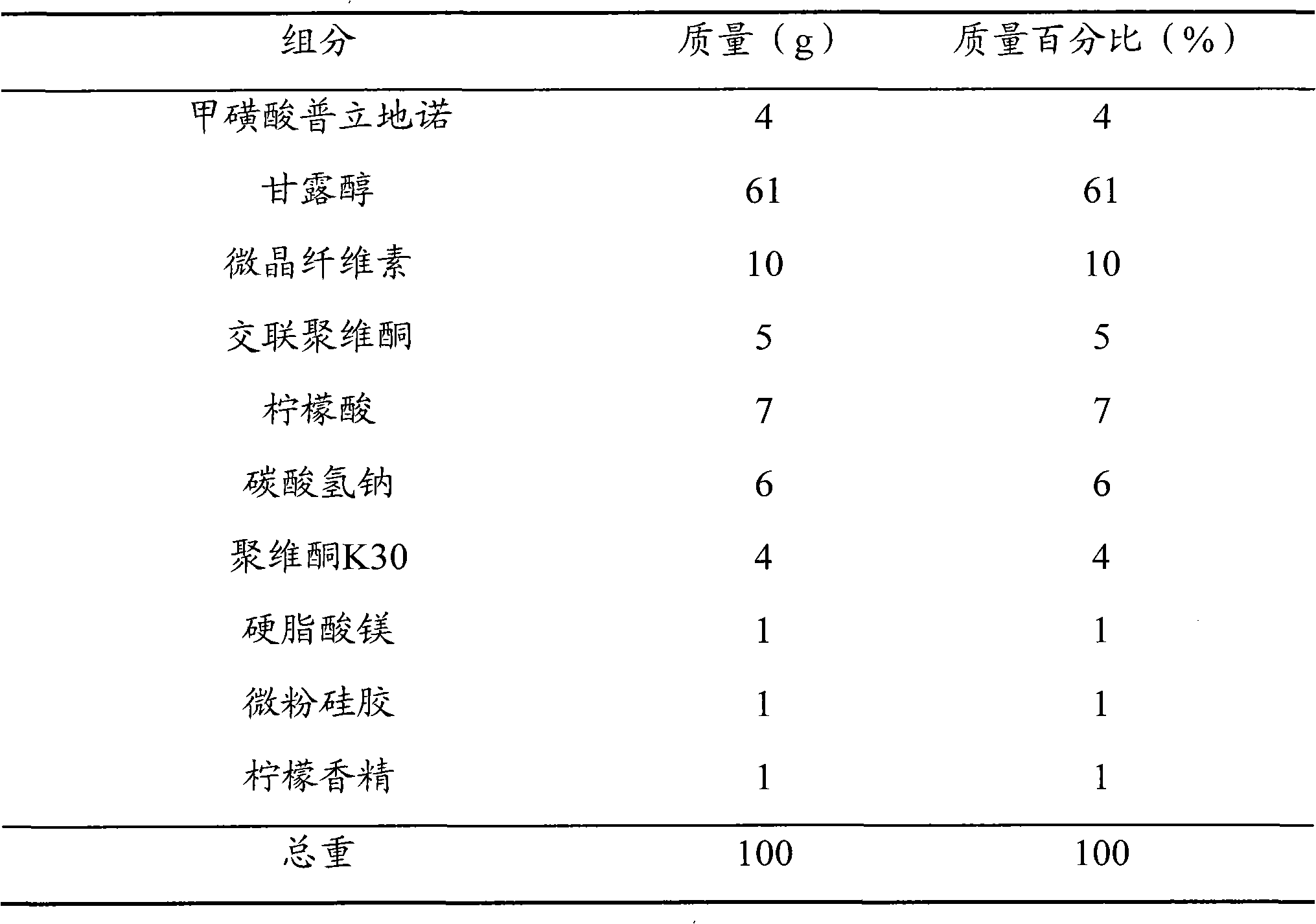
[0048] prescription
[0049]
[0050] method Grind all the components in the prescription into fine powders with a fineness of more than 100 meshes; add 4 g of pritinol mesylate, 61 g of mannitol, 10 g of microcrystalline cellulose, and 2.5 g of crospovidone by equal volume addition method , 7 g of citric acid and 1 g of lemon essence are mixed evenly, adding a mass fraction of 4% povidone K30 solution to make a soft material, granulating with a 24-mesh sieve, drying at 60 ° C, and sizing the dry granules through a 24-mesh sieve; Add 2.5g of crospovidone, 6g of sodium bicarbonate, 1g of magnesium stearate and 1g of micropowder silica gel to the granules, mix well, and press into tablets to make 1000 orally disintegrating tablets of pridinol mesylate, each Contains pridinol mesylate 4mg.
[0051] quality The obtained orally disintegrating tablet has a smooth and beautiful surface; it completely disintegrates in 33 seconds in water at 37±1°C and passes through a 30-...
Embodiment 2
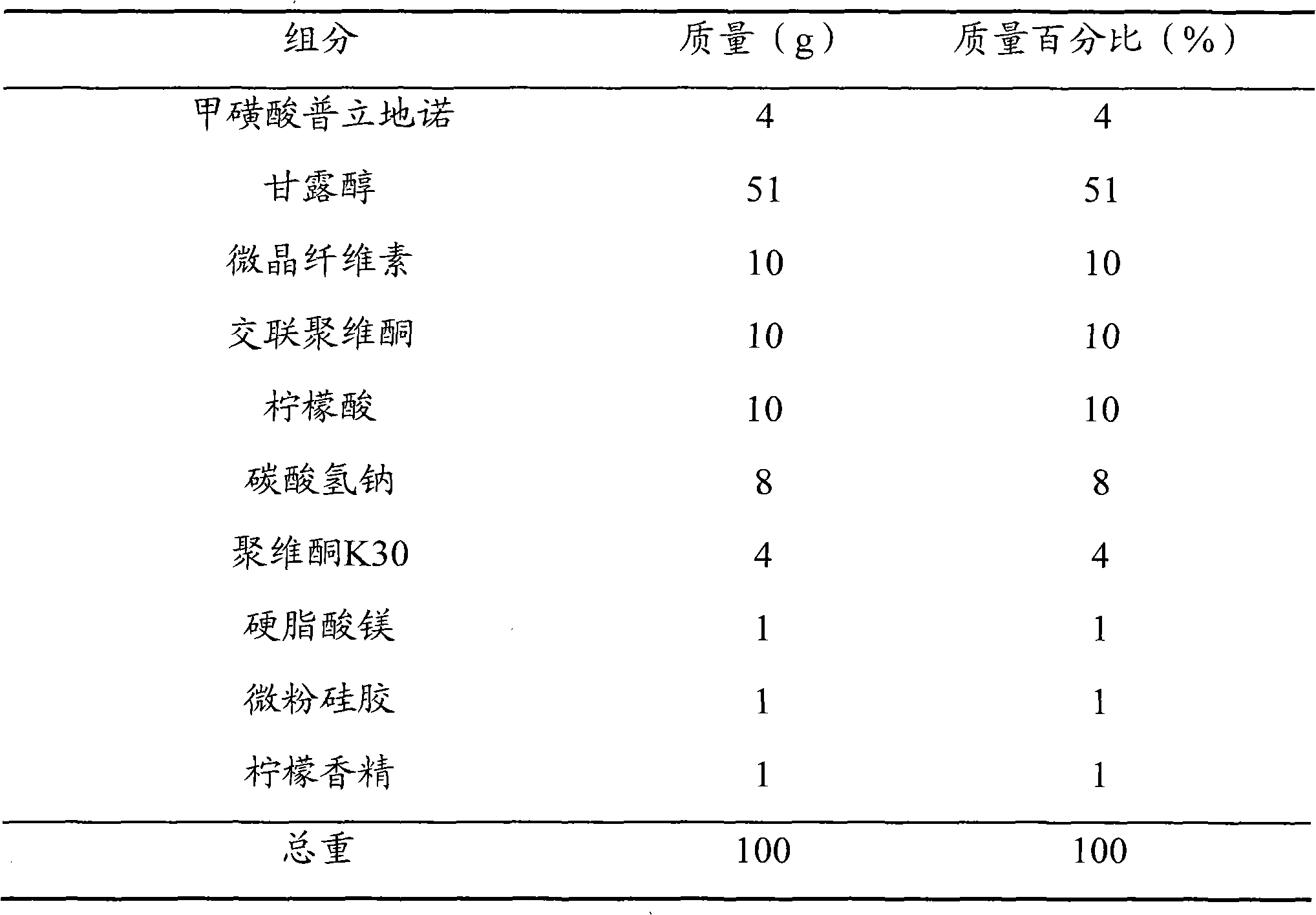
[0053] prescription
[0054]
[0055] method All the components in the prescription were crushed into fine powders with a fineness above 100 meshes; 4 g of pritinol mesylate, 51 g of mannitol, 10 g of microcrystalline cellulose, 5 g of crospovidone, Mix 10 g of citric acid and 1 g of lemon essence, add povidone K30 solution with a mass fraction of 4% to make a soft material, granulate with a 24-mesh sieve, dry at 60 ° C, and pass the dry granules through a 24-mesh sieve for granulation; Add 5g of crospovidone, 8g of sodium bicarbonate, 1g of magnesium stearate and 1g of micropowder silica gel, mix well, and press into tablets to make 1000 orally disintegrating tablets of pridinol mesylate, each containing formazan Pridinol sulfonate 4 mg.
[0056] quality The obtained orally disintegrating tablet has a smooth and beautiful surface; it completely disintegrates in water at 37±1°C within 29 seconds and passes through a 30-mesh sieve; its hardness is 20-45 Newtons; it...
Embodiment 3
[0058] prescription
[0059]
[0060] method Grind all the components in the prescription into fine powders with a fineness of more than 100 mesh; add 4 g of pritinol mesylate with 41 g of mannitol, 10 g of microcrystalline cellulose, and 7.5 g of crospovidone , 13g of citric acid and 1g of lemon essence are mixed evenly, adding a mass fraction of 4% povidone K30 solution to make a soft material, granulating with a 24-mesh sieve, drying at 60°C, and sizing the dry granules through a 24-mesh sieve; Add 7.5g of crospovidone, 10g of sodium bicarbonate, 1g of magnesium stearate and 1g of micropowder silica gel to the granules, mix well, and press into tablets to make 1000 orally disintegrating tablets of pridinol mesylate, each Contains pridinol mesylate 4mg.
[0061] quality The obtained orally disintegrating tablet has a smooth and beautiful surface; it completely disintegrates in 26 seconds in water at 37±1°C and passes through a 30-mesh sieve; the hardness is 20-4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com