Bisglyoxaline organic phosphine compound and preparation method thereof
A biimidazole and compound technology is applied in the field of biimidazole organic phosphine derivative compounds and their synthesis, and achieves the effects of great significance, avoidance of loss and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
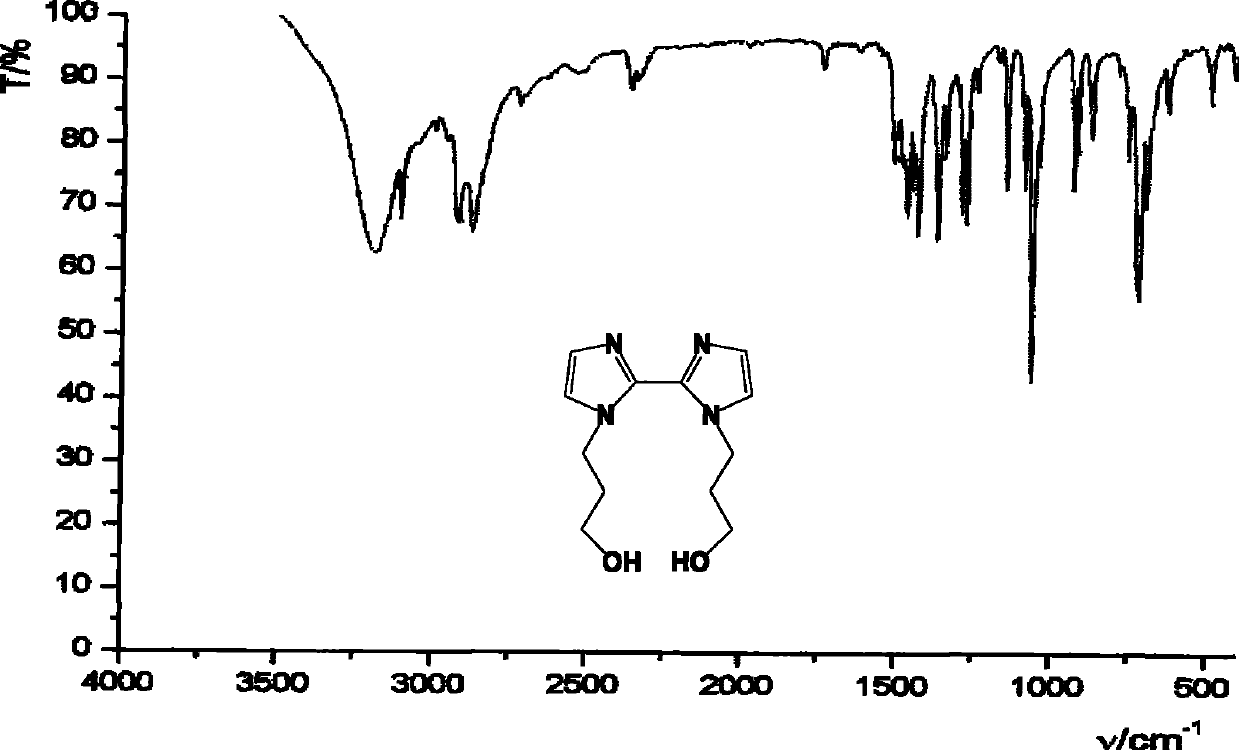
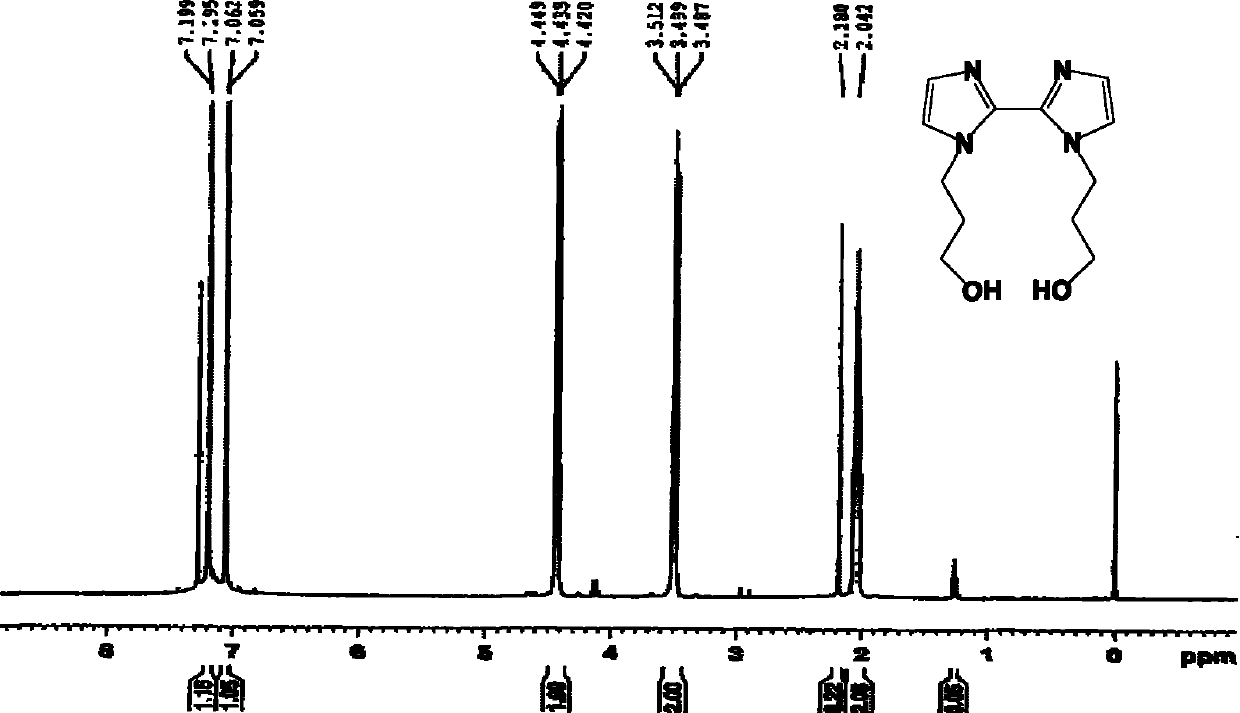
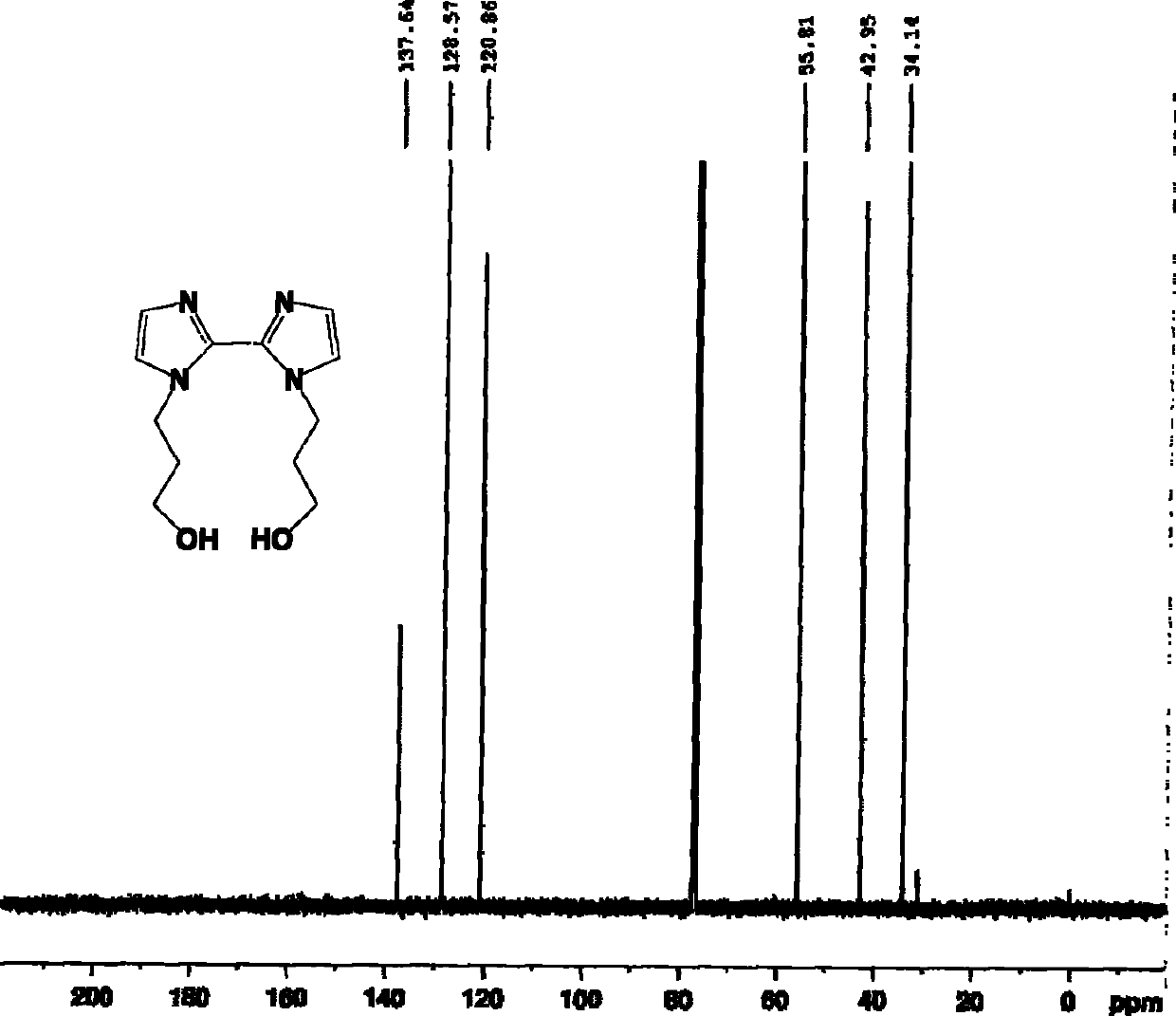
[0070] Take 0.8g (2.4mmol) of 1,1'-bis(ethyl propionate)-2,2'-biimidazole and dissolve it in 30ml of absolute ethanol. Add 0.9 g (24 mmol) sodium borohydride in batches under stirring; heat to reflux for 3 hours. The solvent was removed by rotary evaporation to obtain a white solid, which was dissolved in 3ml of water to obtain a colorless and transparent solution; the solution was adjusted to pH 8-9 with 1M hydrochloric acid solution, and the white turbidity appeared, and it was filtered to obtain a colorless and transparent solution; the solution was 5ml×3 Extract with dichloromethane, and combine the organic phases. The organic phase was dried with anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and petroleum ether and ethyl acetate were recrystallized to obtain 0.53 g of white solid 1,1'-bis(3-hydroxypropyl)-2,2'-biimidazole, the yield : 89%. Melting point: 105-106°C. IR (KBr, cm -1 ) 3190 (br), 2874, 1466, 1429, 1367, 1273, 1146, 1063, 930,...
Embodiment 2
[0073] Take 1.5g (3.8mmol) of 1,1'-bis(butyl propionate)-2,2'-biimidazole and dissolve it in 60ml of absolute ethanol. Add 1.4 g (38 mmol) sodium borohydride in batches under stirring; heat to reflux for 3 hours. The solvent was removed by rotary evaporation to obtain a white solid, which was dissolved in 3ml of water to obtain a colorless and transparent solution; the solution was adjusted to pH 8-9 with 1M hydrochloric acid solution, and the white turbidity appeared, and it was filtered to obtain a colorless and transparent solution; the solution was 10ml×3 Extract with dichloromethane, and combine the organic phases. The organic phase was dried with anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and petroleum ether and ethyl acetate were recrystallized to obtain 0.8 g of 1,1'-bis(3-hydroxypropyl)-2,2'-biimidazole as a white solid. The yield was : 83%. The spectrogram is the same as example 1.
Embodiment 3
[0075]Under an inert atmosphere, 2 g (6.5 mmol) of 1,1'-bis(methyl propionate)-2,2'-biimidazole was dissolved in 40 ml of anhydrous tetrahydrofuran. Add 1.5 g (39.5 mmol) lithium aluminum hydride in batches under stirring; heat to reflux for 5 hours. The solvent was removed by rotary evaporation, 20ml of water and 20ml of dichloromethane were added, the organic phase was separated after suction filtration, the aqueous solution was extracted with dichloromethane, and the organic phases were combined. The organic phase was dried with anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and petroleum ether and ethyl acetate were recrystallized to obtain 0.7g of white solid 1,1'-bis(3-hydroxypropyl)-2,2'-biimidazole, the yield : 44%. The spectrogram is the same as example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com