Novel fusion protein for human HMGB1 A box and acidic tail and use thereof
A fusion protein and acidic technology, applied in the field of genetic engineering, can solve the problems of failing to improve the survival rate of patients, and achieve the effects of avoiding inflammatory effects, strong anti-inflammatory activity, and inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] 1. Cloning of human HMGB1 cDNA
[0080] 1. Isolation of Human Peripheral Blood Mononuclear Cells
[0081] Take 15ml of human peripheral anticoagulant blood (purchased from the Blood Transfusion Department of Southwest Hospital), add sterile PBS to dilute and mix evenly; draw the diluted blood, and carefully add an equal volume of human lymphocyte separation solution along the tube wall. Centrifuge horizontally at 2000r / min for 15 minutes. Carefully suck out the liquid containing human peripheral blood mononuclear cells in the white mist in the middle layer, wash with sterile PBS twice, and centrifuge at 1000r / min for 15 minutes each time.
[0082] 2. Extraction of total RNA from human peripheral blood mononuclear cells
[0083] Use the Tripure kit to extract total cellular RNA, and follow the instructions: 1ml / 5~10×10 6 Add an appropriate amount of Tripure to each cell, invert the Ep tube to fully mix the Tripure and the cells, and place at room temperature for 5 min...
Embodiment 2
[0104] 1. Cloning of human HMGB1 A box and acidic tail flexible junction coding sequence
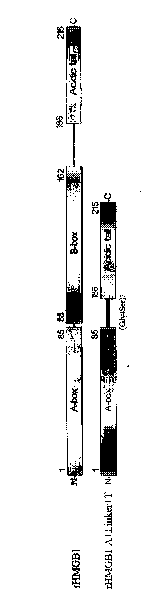
[0105] 1. Schematic diagram of the fusion protein structure of human HMGB1 A box and acidic tail flexible linker figure 2 .
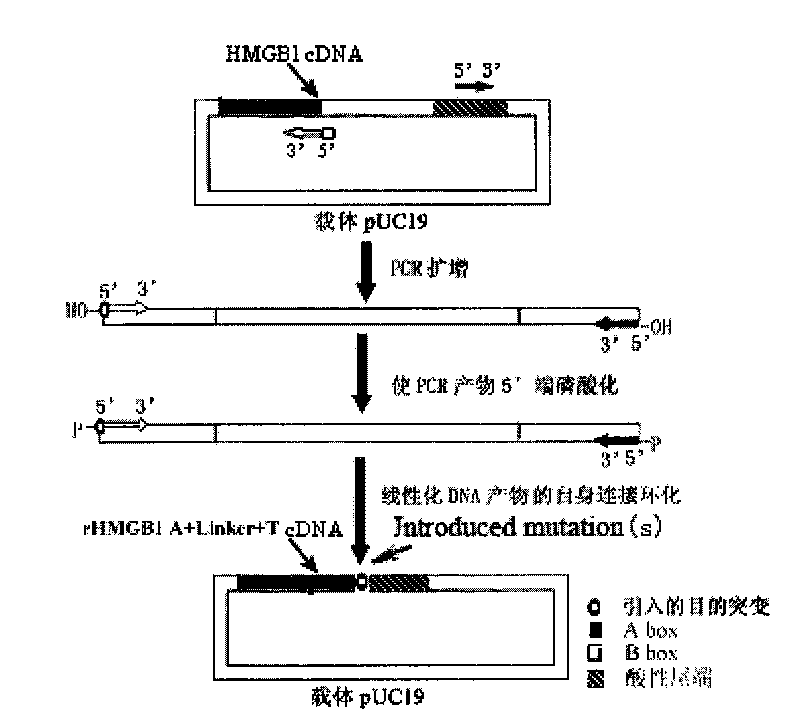
[0106] 2. One-step inverse PCR mutagenesis strategy to amplify human HMGB1 A box and acidic tail rigid junction and flexible junction coding sequence respectively
[0107] (1) The basic principle of one-step reverse PCR mutagenesis strategy: design a pair of primers with adjacent 5' ends and opposite directions at the 3' ends to introduce mutation points; in order to ensure the correctness of the amplified product sequence, use high-fidelity DNA polymerase Pyrobest DNA Polymerase was used to amplify; in the same reaction system, the end of the PCR product was smoothed and the 5' end was phosphorylated; the product was then subjected to self-ligation reaction; finally, plasmid DNA transformation and mutant screening were performed. (For specific principles, see ...
Embodiment 3
[0188] 1. The target protein removes LPS
[0189] 1. Detoxi-Gel TM Endotoxin Removing Gel removes LPS in the target protein (refer to Pierce company instructions)
[0190] (1) Detoxi-Gel placed at 4°C TM Endotoxin Removing Gel column was left at room temperature for 30 minutes.
[0191] (2) Remove the cap from the bottom of the column, and add 5 times column volume of 1% sodium deoxycholate to regenerate Detoxi-Gel.
[0192] (3) Add 5 times the column volume of sterile pyrogen-free water to wash the column.
[0193] (4) Add 5 column volumes of sterile pyrogen-free water to equilibrate Detoxi-Gel.
[0194] (5) Cover the cap at the bottom of the column, and each protein of interest is passed through Ni 2+ -NTA column purification collected Elution Buffer and dialyzed liquid respectively Detoxi-Gel TM Endotoxin Removing Gel column, at room temperature for 1 hour.
[0195] (6) Remove the cap at the bottom of the column, wash the column with sterile pyrogen-free water, co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com