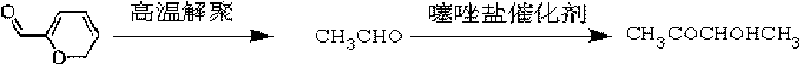Method for synthesizing 3-hydroxy-2-butanone
A technology of hydroxyl and butanone, which is applied in the field of synthesizing 3-hydroxy-2-butanone, can solve the problems of acetaldehyde increasing industrial costs, high cost or technical requirements, difficult transportation and storage, etc., so as to reduce the loss of raw materials and transportation Effects of storage problems, simplified process flow, convenient transportation and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
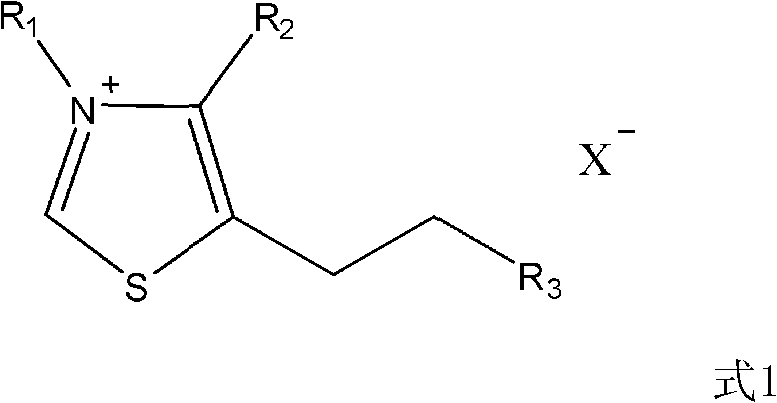
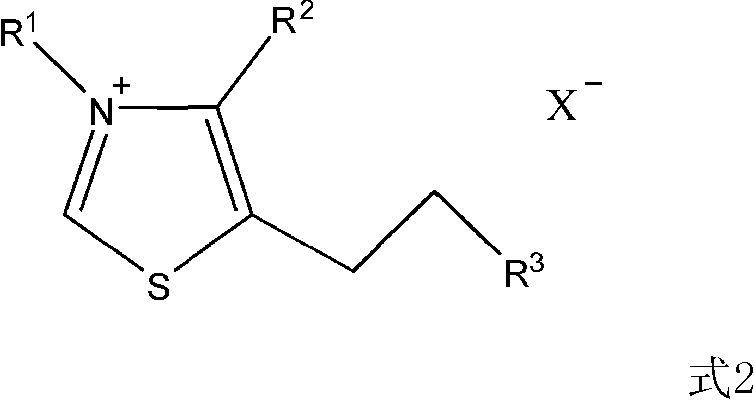
[0018] Preparation of thiazole salts
[0019] According to the literature (Hermann, Stetter.Angew.Chem.Int.Engl.15(1976)639-647), using the corresponding thiazole raw materials and corresponding halogenated hydrocarbons, the following two thiazole salt catalysts were synthesized
[0020]
Embodiment 1
[0022] Get 20g paraldehyde and 0.2g thiazole salt (I) (1wt%) and join in the autoclave together, adjust the pH value of the reaction mixture to 9-10 with 2M sodium hydroxide solution, first extract the reactor In the air, stirred and slowly heated to 140 ° C, reacted for 3 hours, naturally cooled after the reaction, the product was analyzed by gas chromatography, the conversion rate of paraldehyde was 91%, and the selectivity of 3-hydroxy-2-butanone was 92%. %.
Embodiment 2
[0024] Get 20g paraldehyde and 0.2g thiazole salt (I) (1wt%) and join in the autoclave together, adjust the pH value of the reaction mixture to 9-10 with 2M sodium hydroxide solution, first extract the reactor In the air, stirred and slowly heated to 150 ° C, reacted for 3 hours, naturally cooled after the reaction, the product was analyzed by gas chromatography, the conversion rate of paraldehyde was 94%, and the selectivity of 3-hydroxy-2-butanone was 95%. %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com