Isopentenyl-oxyl substituted dehydrogenized silybin ether and preparation method and application thereof
A technology of isopentenyloxy and silibinin, which is applied in the field of preparation of dehydrosilibinin ether derivatives, can solve problems such as damage and changes in the biphospholipid layer of cell membranes, and achieves a simple method and free radical scavenging , the effect of protecting brain nerve cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
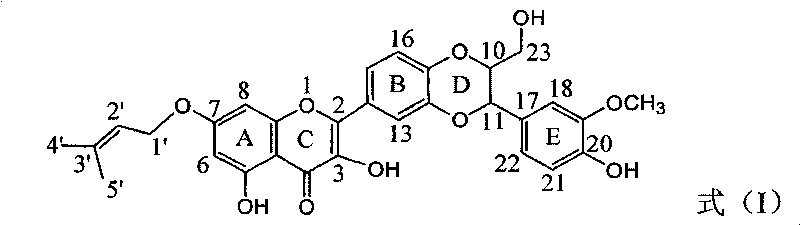
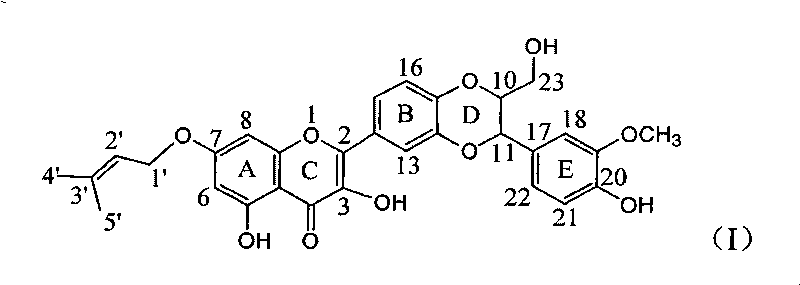
[0023] Example 1 : Compound I is the preparation of 7-prenyloxy substituted deoxysilibinin ether
[0024]
[0025] In a dry reaction flask, 0.241 g of silibinin was dissolved in 5 ml of DMF, 0.276 g of potassium carbonate was added, and stirred for 10 minutes to dissolve completely. 50 mg of isopentenyl bromide was slowly added dropwise, stirred for 5 minutes, and reacted at 75°C for 1 hour. Stand to cool, add 20 ml of distilled water, extract with ethyl acetate three times (10 ml each time), combine the organic layers, wash with 10 ml of distilled water, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. A light yellow crude product was obtained, which was subjected to 200-300 mesh silica gel (7 g) column chromatography, eluting with petroleum ether: ethyl acetate: acetic acid = 5:1:0.1 to obtain 39.3 mg of a light yellow solid. Yield 14.3%.
[0026] Compound I: 2-[2,3-Dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-hydroxymethyl-1,4-benzodioxane-6-]- 3...
Embodiment 2
[0031] Example 2 : Activity test of compound I for scavenging diphenylpicrylhydrazyl free radicals (1,1-diphenyl-2-picrylhydrazyl, DPPH) in vitro
[0032] 2.1 Test principle: DPPH is an aryl radical that can exist stably in vitro and is widely used to evaluate the antioxidant activity of compounds. free radical scavenging ability. DPPH is easily soluble in methanol, the solution is dark purple, and has a maximum absorption at 517nm. If the free radical is captured by the compound to be tested, the absorbance at 517nm will decrease. The more the absorbance decreases, the greater its ability to scavenge DPPH free radicals Also stronger.
[0033] 2.2 Experimental materials and samples
[0034] 2.2.1 Experimental reagents:
[0035] 2.2.1.1 Phenazine methosulfate (PMS), nitroblue tetrazolium (NBT), and phenanthrozine (ferrozine) were purchased from Sigma;
[0036] 2.2.1.2 Silybin was purchased from Liaoning Panjin Tianyuan Pharmaceutical Co., Ltd., with a purity of 98% by HPL...
Embodiment 3
[0054] Example 3 : formula (I) compound I to hydrogen peroxide H 2 o 2 Protective activity test of induced PC12 cell injury
[0055] 3.1 Experimental materials and samples
[0056] 3.1.1 Cells: Rat adrenal pheochromoma PC12 cells were purchased from Shanghai Cell Institute, Chinese Academy of Sciences.
[0057] 3.1.2 Experimental reagents:
[0058] 3.1.2.1 Hydrogen peroxide (H 2 o 2 ), nitrobluetetrazolium (NBT), phenanthrozine (ferrozine) were purchased from Sigma Company;
[0059] 3.1.2.2 Silybin was purchased from Liaoning Panjin Tianyuan Pharmaceutical Co., Ltd., and its purity was 98% by HPLC.
[0060] 3.1.2.3 Tris base, DMEM medium was purchased from Gibco;
[0061] 3.1.2.4 MTT was purchased from Amresco;
[0062] 3.1.2.5 Calf serum was purchased from Hangzhou Sijiqing Bioengineering Materials Co., Ltd.;
[0063] 3.1.2.6 Penicillin and streptomycin are produced by Shijiazhuang Pharmaceutical Group Co., Ltd.;
[0064] 3.1.2.7 Other reagents are domestic analyt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com