Super-biparental self-cleaning coating material and preparation method thereof
A self-cleaning coating and self-cleaning technology, applied in coatings and other directions, can solve the problems of easy powdering of self-cleaning coatings, low self-cleaning efficiency of coatings, shortened service life of coatings, etc., and achieve outstanding self-cleaning properties , no loss of mechanical properties, and good weather resistance of the coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Mix tetraethyl orthosilicate with 100 parts of γ-glycidyloxypropyltrimethoxysilane (mass ratio: 95 / 5), 87.5 parts of absolute ethanol, and 45.0 parts of deionized water, and mix them in 0.24 parts of hydrochloric acid Under the action of catalysis, hydrolyze and condense at 70° C. for 3 hours to obtain siloxane oligomer (AI).
[0030] 45 parts of butyl acrylate, 45 parts of styrene, 10 parts of γ-(methacryloyloxy)propyltrimethoxysilane, 3 parts of γ-mercaptopropyltriethoxysilane, 40 parts of xylene, initiator (LUPEROX 575) 3 parts, the styrene-acrylic oligomer (B) was prepared by semi-continuous solution polymerization (polymerization temperature 120°C).
[0031] Stir and mix 12 parts of styrene-acrylic oligomer B, 12 parts of P251, and 36 parts of xylene, add an appropriate amount of zirconium beads (ball diameter 0.3mm), and disperse at high speed for 4 hours to obtain uniformly dispersed nano-TiO 2 slurry.
[0032] Take 90 parts of siloxane oligomer AI, 10 parts of...
Embodiment 2
[0034] Mix tetraethyl orthosilicate with 100 parts of vinyltrimethoxysilane (mass ratio: 95 / 5), 87.5 parts of absolute ethanol, and 45.0 parts of deionized water. The siloxane oligomer (AII) was obtained by hydrolyzing and condensing for 3 hours.
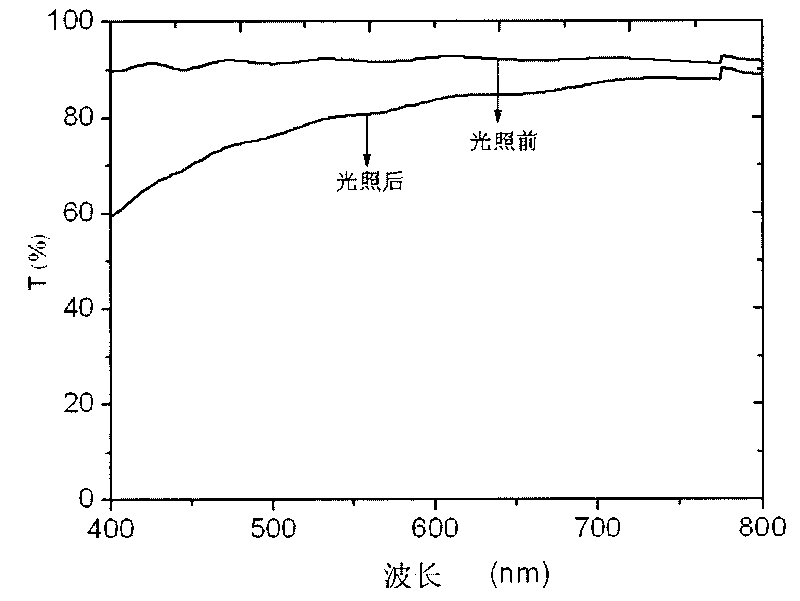
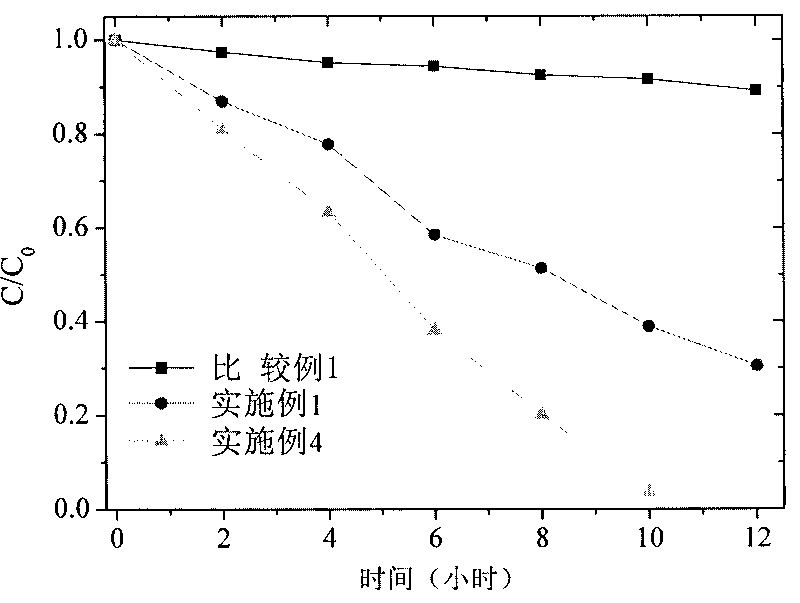
[0035] 90 parts of siloxane oligomer AII, 10 parts of styrene-acrylic oligomer B in embodiment 1, nanometer TiO in embodiment 1 2 Mix 3 parts of the slurry evenly to prepare the coating component A; before coating, add 10 parts of γ-aminopropyltriethoxysilane, and apply a film on the glass sheet by dip coating, and dry it at room temperature for one week. After the coating was placed under the summer sun, the water contact angle and cyclohexane contact angle of the coating decreased to 0° after 14 days. After the weather resistance test, the coating surface was free of powder, and the pencil hardness changed from 6H to 4H.
Embodiment 3
[0037] With 90 parts of 3074 silicone resins, 10 parts of styrene-acrylic oligomer B in embodiment 1, nanometer TiO in embodiment 1 2 Mix 7 parts of the slurry evenly to prepare coating component A; before coating, add 10 parts of γ-aminopropyltriethoxysilane, apply a film on a glass sheet by dip coating, dry at room temperature for one week, and apply After the coating was placed under the summer sun, the water contact angle and cyclohexane contact angle of the coating dropped to 0° after 15 days. After the weather resistance test, the coating surface was free from chalking, and the pencil hardness changed from 4H to 3H.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Water contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com