Gas diffusion electrode and preparation method thereof
A gas diffusion electrode and diffusion layer technology, applied in the direction of electrodes, electrode shape/type, electrolytic components, etc., can solve the problems of broken electrodes, high price, lifespan and electrode performance impact, and achieve improved corrosion resistance and conductivity , good corrosion resistance and electrical conductivity, and the effect of avoiding etching damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
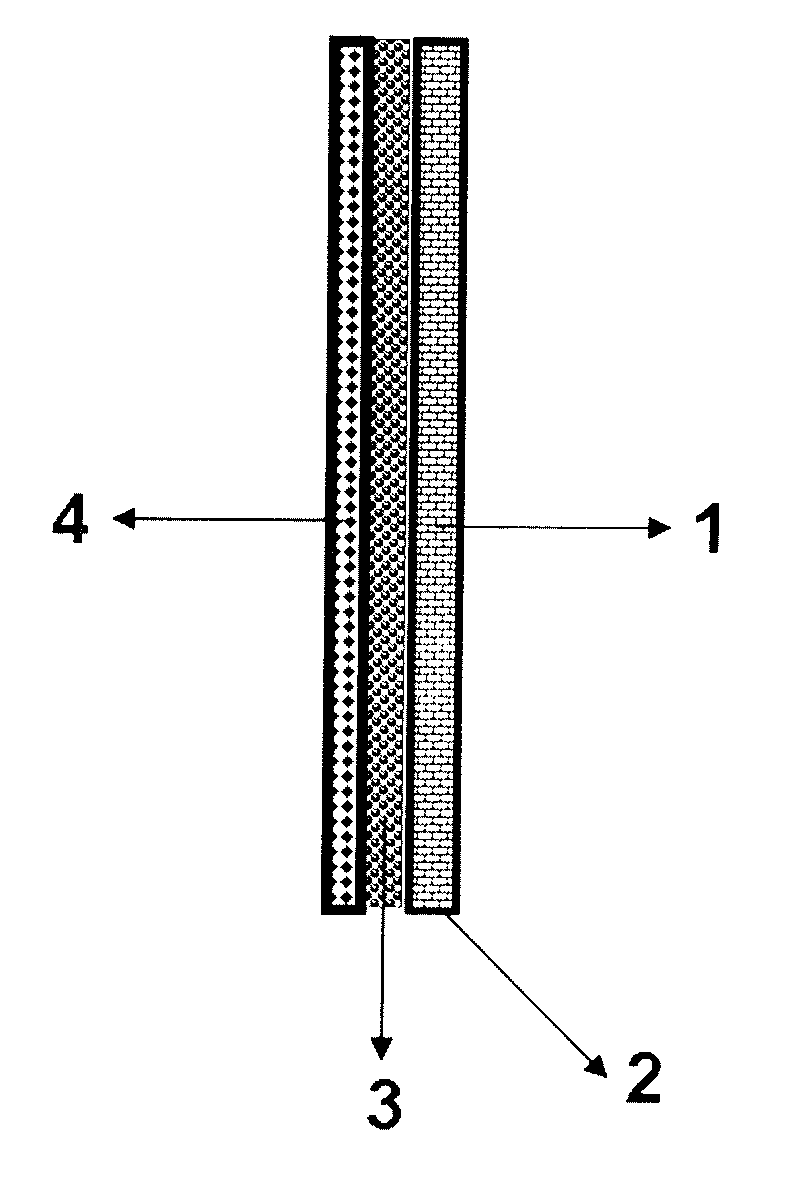
[0020] 1) Weigh 2g of graphitized carbon black and dissolve it in 200ml of isopropanol, then add 0.56ml of polytetrafluoroethylene emulsion (0.84g) with a mass fraction of 60%, stir evenly, and smear it on a 12cm*12cm silver-plated On the nickel foam, after drying at room temperature for 8 hours, press on a hot press at 1tf and 20°C for 60s to obtain diffusion layer 1 and current collector 2;
[0021] 2) buckle a silver-plated nickel mesh of 12cm*12cm on the current collector 2 as a support, and then press it on a hot press at 1tf and 20°C for 60s to complete the production of the support 3;
[0022] 3) the silver-carbon catalyst 1g and 0.75g acidified graphitized carbon black of silver loading rate 40% are dissolved in 100ml Virahol, then add 0.83ml mass fraction and be 60% polytetrafluoroethylene emulsion (1.25 g) After stirring evenly, apply it on the support body 3, dry it at room temperature for 8 hours, and press it on a hot press at 3tf and 360° C. for 60 seconds to obt...
Embodiment 2
[0024] 1) Weigh 1.4g of graphitized carbon black and dissolve it in 100ml of isopropanol, then add 0.17ml of polytetrafluoroethylene emulsion (0.26g) with a mass fraction of 60%, stir evenly, and smear it on a 12cm*12cm plated On silver nickel foam, after drying at room temperature for 7 hours, press on a hot press at 2tf and 20°C for 120s to obtain diffusion layer 1 and current collector 2;
[0025] 2) buckle a silver-plated nickel mesh of 12cm*12cm on the current collector 2 as a support, and then press it on a hot press at 2tf and 20°C for 120s to complete the production of the support 3;
[0026] 3) the silver-carbon catalyst 2g and 1.5g acidified graphitized carbon black of silver loading rate of 40% are dissolved in 200ml Virahol, then add 0.97ml mass fraction and be 60% polytetrafluoroethylene emulsion (1.46 g) After stirring evenly, spread it on the support body 3, dry it at room temperature for 7 hours, and press it on a hot press at 4tf, 370° C. for 120 seconds to ob...
Embodiment 3
[0028]1) Weigh 2.5g of graphitized carbon black and dissolve it in 300ml of isopropanol, then add 1.11ml of 60% polytetrafluoroethylene emulsion (1.67g) in parts by mass and stir evenly, then smear it on a 12cm*12cm silver-plated On the nickel foam, after drying at room temperature for 9 hours, press on a hot press at 3tf and 20°C for 240s to obtain diffusion layer 1 and current collector 2;
[0029] 2) Fasten a 12cm*12cm silver-plated nickel mesh as a support on the current collector 2, and then press it on a hot press at 3tf and 20°C for 240s to complete the production of the support 3;
[0030] 3) the silver-carbon catalyst 3g and 2.25g acidified graphitized carbon black of silver loading rate of 40% are dissolved in 400ml Virahol, then add 0.64ml mass fraction and be 60% polytetrafluoroethylene emulsion (0.97 g) After stirring evenly, spread it on the support body 3, dry it at room temperature for 9 hours, and press it on a hot press at 6tf and 380° C. for 240 seconds to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com