Process for producing aromatic polyene antibiotic through fermentation
A technology for aromatic polyenes and antibiotics, applied in the field of fermentation, can solve the problems of restricting the clinical trials and industrialization of aromatic polyene antibiotics, low yield of aromatic polyene antibiotics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
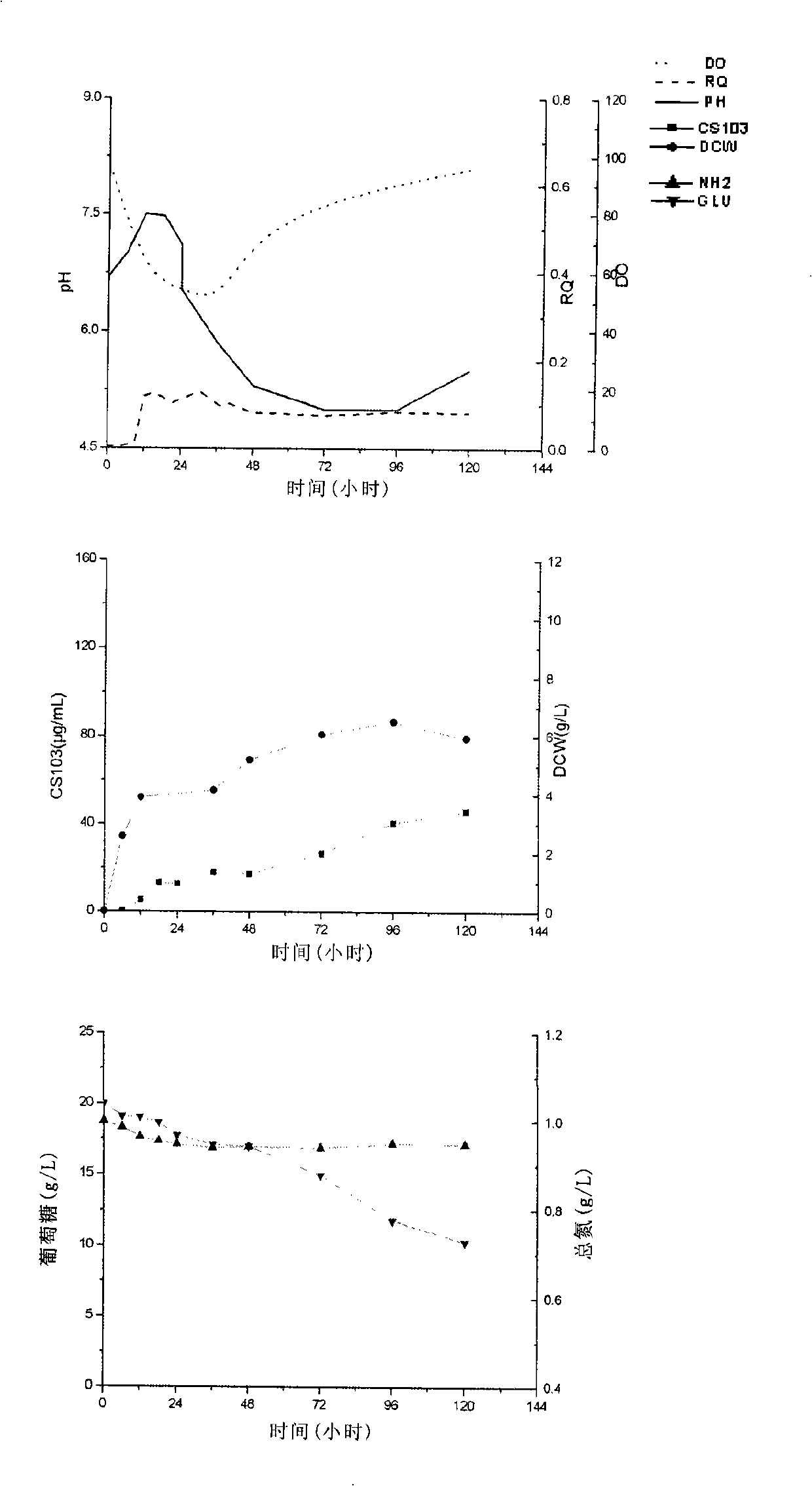
[0064] In order to determine the time to regulate the pH, the concentration of the aromatic polyene antibiotics in the fermentation broth can be measured regularly (such as every 5 minutes or every 10 minutes) or in real time during the fermentation process, so as to judge whether the engineering bacteria start to produce aroma. polyene antibiotics. Once the engineered bacteria start or are about to start producing aromatic polyene antibiotics, the pH can be adjusted to 6.4-7.4.
[0065] Taking FR-008 decarboxylation-derived polyketide antibiotic CS103 as an example, the following methods were used to detect the initial stage of fermentation: high performance liquid chromatography (Agenlent 1100), the chromatographic column is SB-C8 (4.6mm*250mm), and the mobile phase is 20mM, pH4. 6 Ammonium acetate buffer solution-acetonitrile (60:40), the flow rate is 1mL / min, the column temperature is 25°C, the detection wavelength is 380nm, and the regression equation is obtained under th...
Embodiment 2
[0071] Fermentation of Polyketide Antibiotic CS103 Derivatized by Decarboxylation of FR-008
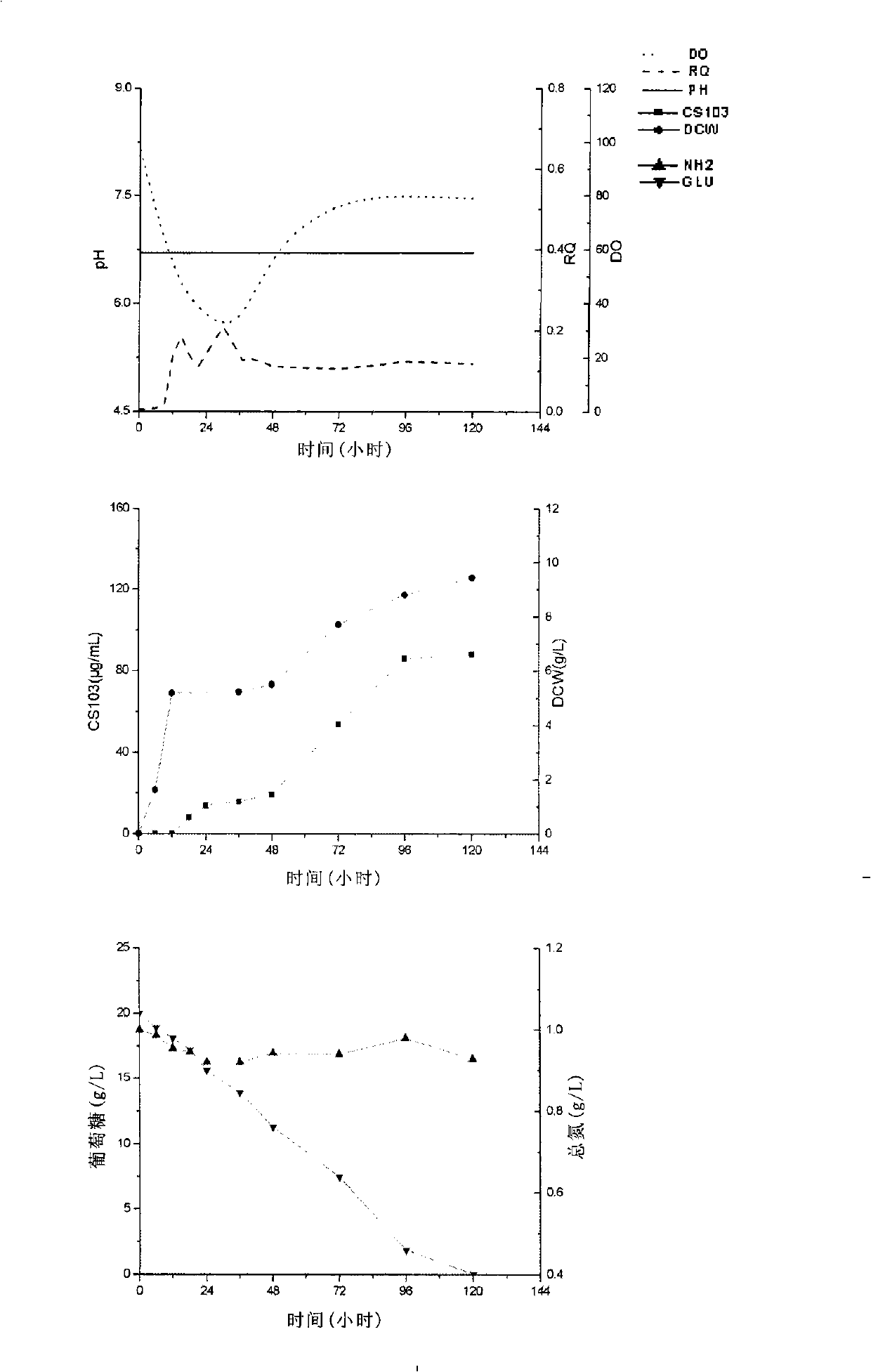
[0072] Same as Example 1, the difference lies in: maintain pH6.9 with hydrochloric acid and sodium hydroxide after inoculation, cultivate for 120 until the end of fermentation.
[0073] The result is as figure 2 shown. When the fermentation process maintains a pH of 6.9, due to the relatively stable pH of the fermentation process, the growth ability of the bacteria is also improved, and the final dry cell weight reaches 9.4g / L. Substrate consumption rate is quickened, and glucose is consumed to 10g / L in 48-72h, and 120h is almost exhausted, and does not control the batch fermentation of pH in embodiment 1, just consumes to about 10g / L until 120h. The consumption of amino nitrogen is also significantly accelerated. It can be seen that the metabolic rate of the bacteria is accelerated, which can also be clearly reflected from the dissolved oxygen and RQ values. The minimum value of...
Embodiment 3
[0075] Fermentation of Polyketide Antibiotic CS103 Derivatized by Decarboxylation of FR-008
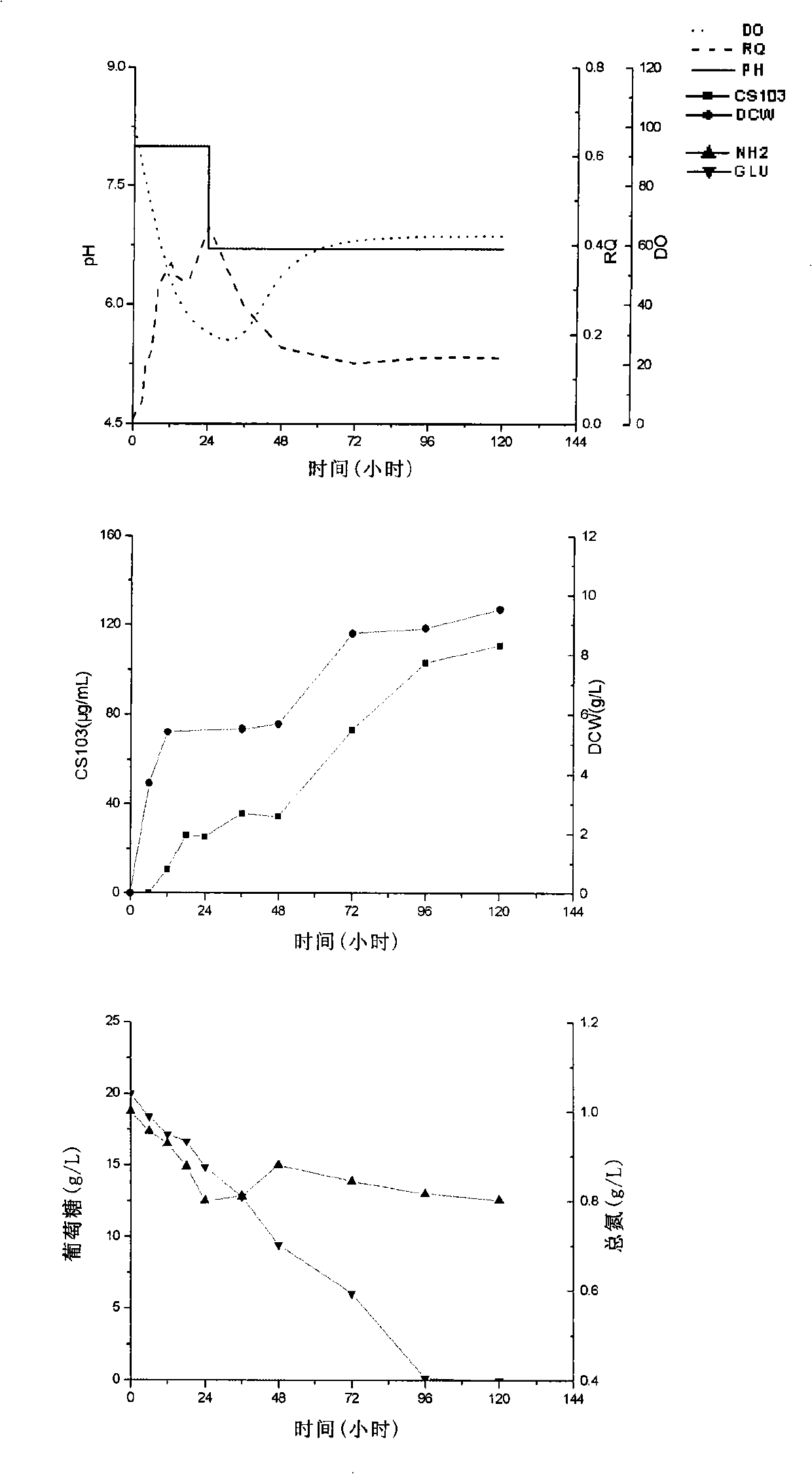
[0076] Same as Example 1, the difference lies in: after inoculation, the pH was maintained at 8.0 with hydrochloric acid and sodium hydroxide. After 24 hours, the pH was quickly adjusted to 6.9, and maintained until the end of fermentation.
[0077] The result is as image 3 shown. After adopting the strategy of controlling the pH in stages, cultivated for 120h, put the fermentation level of FR-008 decarboxylated polyketide antibiotic CS103 into the bottle to further increase to 111 μg / mL, which was 141% higher than that in the batch fermentation without pH control in Example 1. Controlled pH6.9 batch fermentation also improved 26% among the example 2.
[0078] In addition, the highest bacterial dry weight DCW Max It was 9.5g / L, and there was no significant change from batch fermentation with controlled pH6.9. This indicated that the increase in antibiotic production was not due t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com