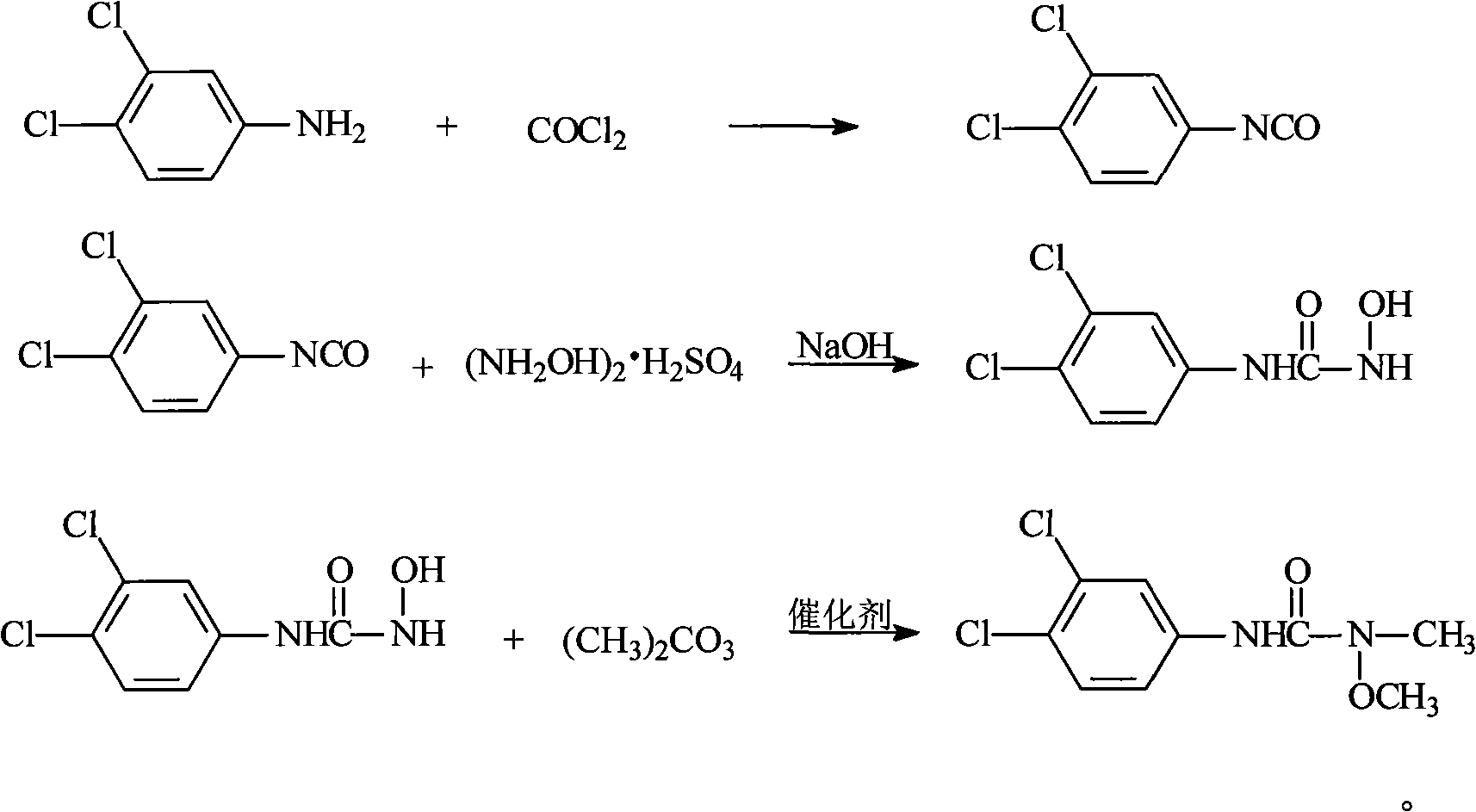
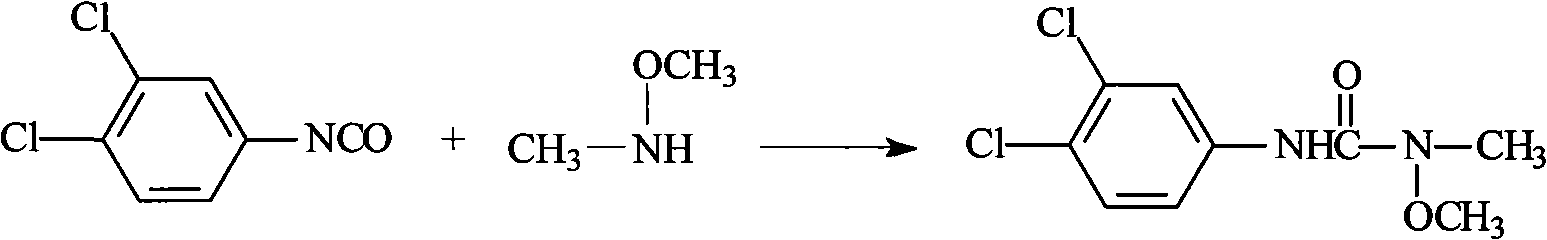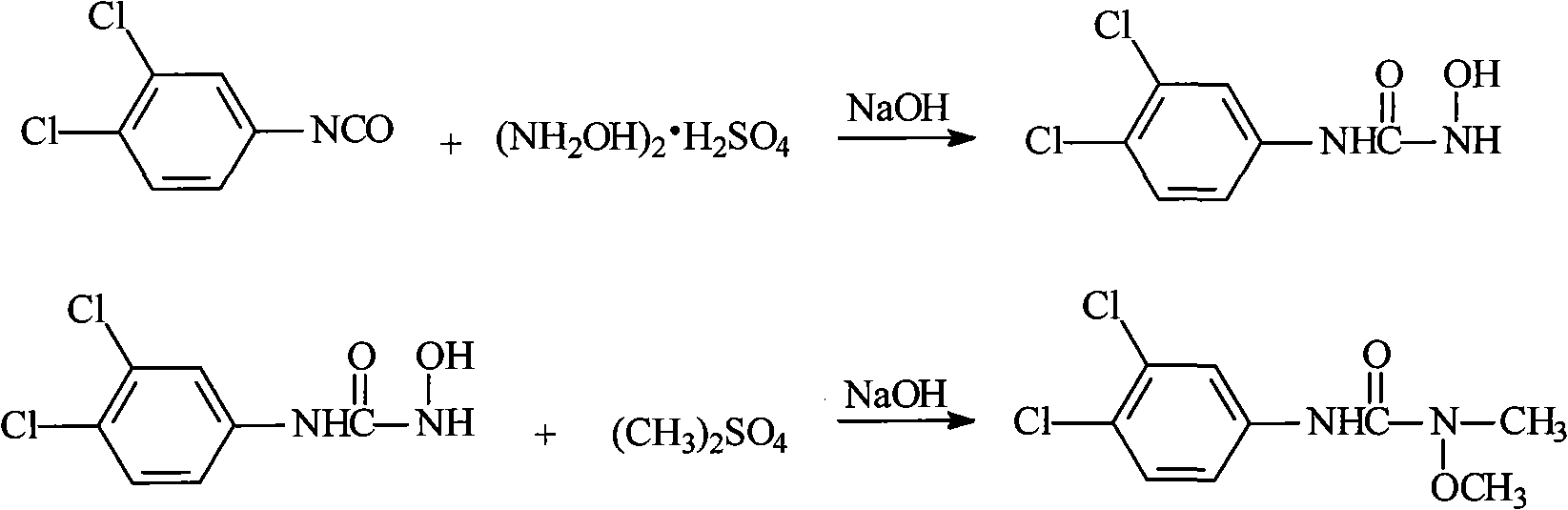Method for preparing 3-(3,4-dichlorophenyl)-1-methoxy-1-methyl urea
A technology of dichlorophenyl and dichlorophenyl isocyanate, applied in the field of preparation of 3--1-methoxy-1-methylurea, which can solve the problems of low manufacturing cost, difficult treatment of salty wastewater, unsafe production, etc. problems, to achieve the effect of excellent quality, less three wastes, and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Add 250ml of toluene and 50g of 3,4-dichloroaniline to the flask, stir to raise the temperature, reflux for dehydration, and obtain 3,4-dichloroaniline toluene solution for later use.
[0032] 2. Take another flask, add 250ml of toluene to it, lower the temperature, and pass in phosgene. When the temperature drops to about 0°C, start to add the above-prepared 3,4-dichloroaniline toluene solution dropwise. Enter phosgene for about 2 hours, the flow rate of phosgene is: 200-250ml / min (the amount of phosgene introduced is about 48-60g), and the reaction temperature is controlled below 5°C.
[0033] 3. Then slowly raise the temperature, 4h~5h to 80°C, then keep warm at 80°C~90°C for 1h, the transparent material is the end point of the reaction. After the reaction is completed, use nitrogen to drive phosgene at 80°C to 90°C for 3h to 4h to obtain a toluene solution of 3,4-dichlorophenylisocyanate for later use.
[0034] 4. Pour the toluene solution of 3,4-dichlorophenyli...
Embodiment 2
[0038] The synthesis of 1-hydroxy-3-(3,4-dichlorophenyl)urea is the same as in Example 1.
[0039] Put 30g of dry product of 1-hydroxy-3-(3,4-dichlorophenyl)urea into the flask equipped with rectification tower (ф24mm×600mm), 100ml of solvent diethylene glycol dimethyl ether, dimethyl carbonate 40g, (Bu) 4 NBr / K 2 CO 3 (where (Bu) 4 NBr: 0.5g, K 2 CO 3 : 5g) heat up to 85°C to 90°C for heat preservation reaction, as the reaction proceeds, methanol is continuously generated, and the generated methanol is not rectified, but remains in the reaction vessel. After 6 hours of reaction, the temperature is raised to reflux for 4 hours , after the reaction is over, cool down, filter with suction, distill off the solvent under reduced pressure, then add ethanol (or mother liquor) for recrystallization to obtain 3-(3,4-dichlorophenyl)-1-methoxy-1-methylurea 25.5 g, the content is 90.5%, and the yield is 75.4% (not discounted). Appearance: white solid.
Embodiment 3
[0041] The synthesis of 1-hydroxy-3-(3,4-dichlorophenyl)urea is the same as in Example 1.
[0042] Put 30g of dry product of 1-hydroxy-3-(3,4-dichlorophenyl)urea into the flask equipped with rectification tower (ф24mm×600mm), 100ml of solvent DMF, 40g of dimethyl carbonate, (Bu) 4 NBr / K 2 CO 3 (where (Bu) 4 NBr: 0.5g, K 2 CO 3 : 5g) heat up to 85°C to 90°C for heat preservation reaction, as the reaction proceeds, methanol is continuously generated, and at the same time, the generated methanol is continuously distilled out. After 6 hours of reaction, the temperature is raised to 125°C to 130°C for reaction 4h, after the reaction is over, cool down, filter with suction, distill off the solvent under reduced pressure, then add ethanol (or mother liquor) for recrystallization to obtain 3-(3,4-dichlorophenyl)-1-methoxy-1-methylurea 27.5g, the content is 94.7%, the yield is: 81.4% (not 100%). Appearance: brown solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com