Method for preparing cephapirin benzathine
A technology for benzathine cefapirin and cefapirin acid, which is applied in the field of chemical drug synthesis and achieves the effects of easy operation, simplified steps and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
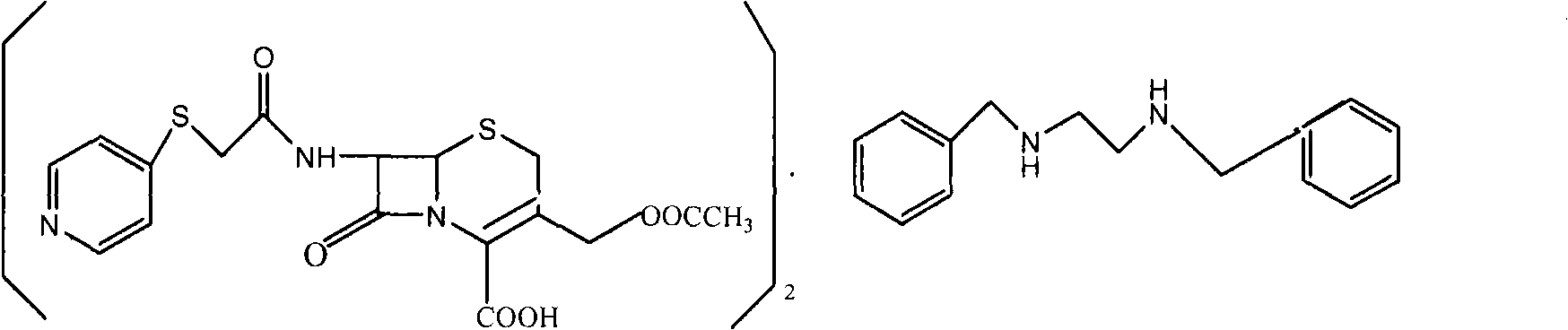
[0023] The preparation of embodiment 1 benzathine cefapirin
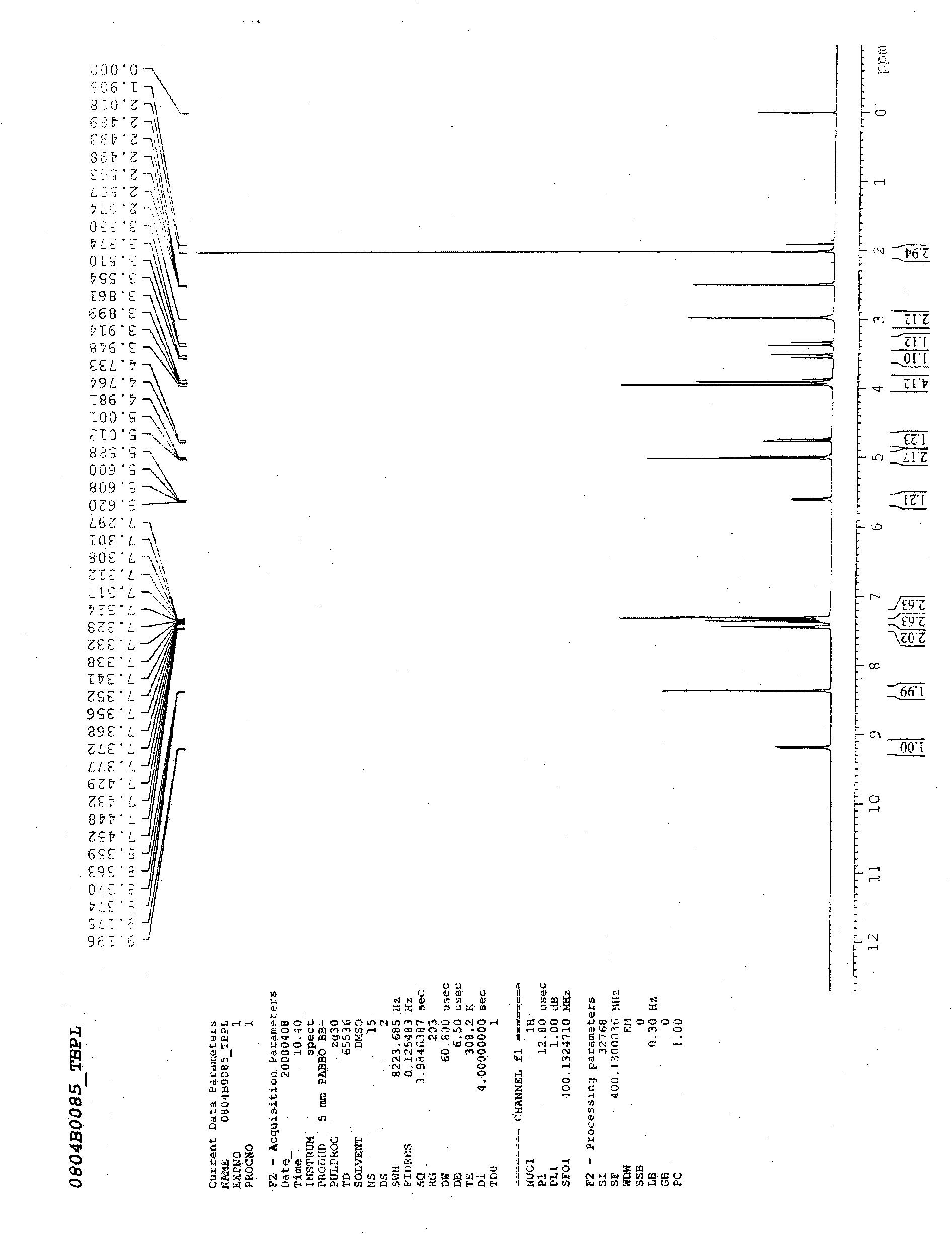
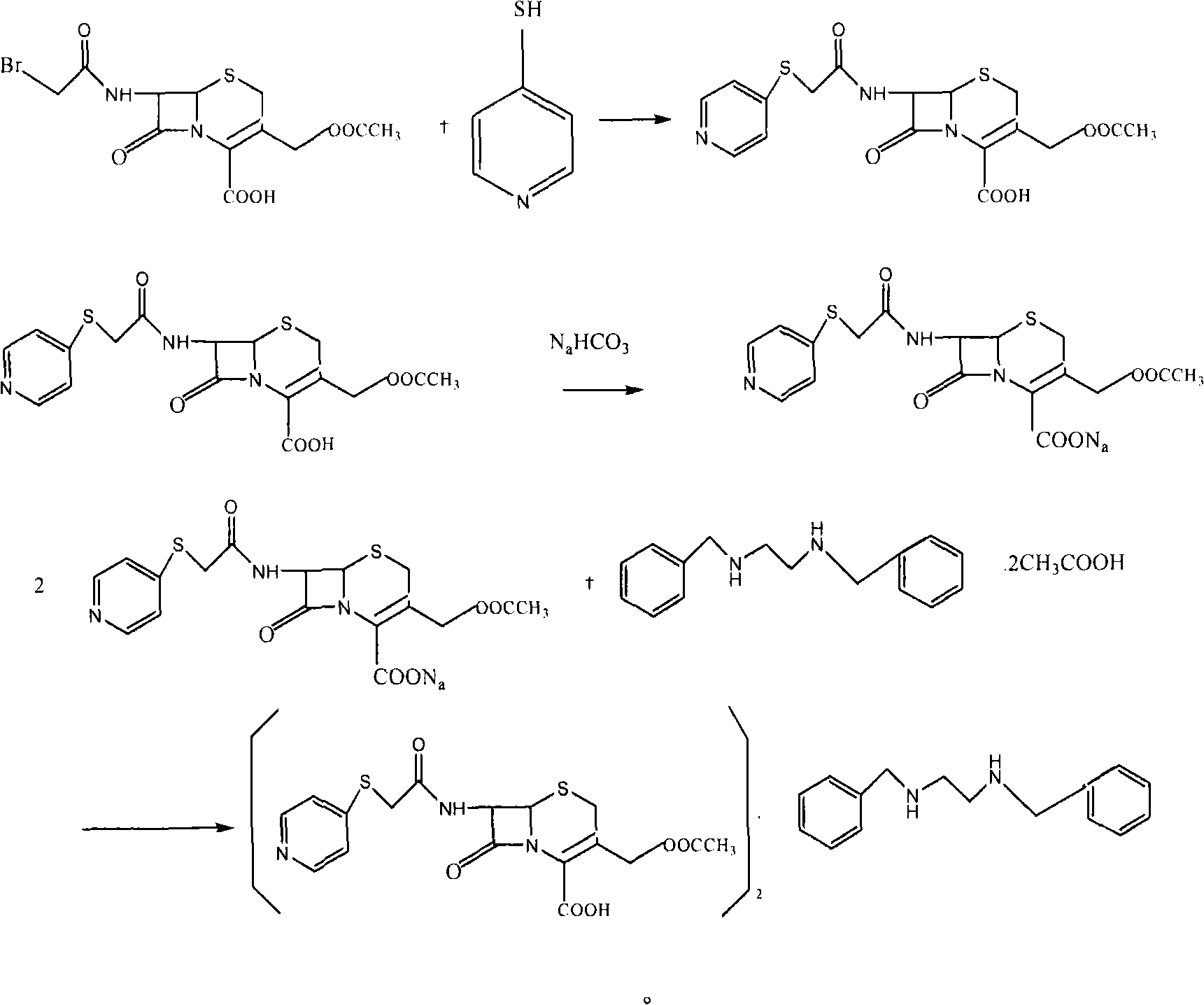
[0024] Add 1003 milliliters of acetone, 783 milliliters of methanol and 391 milliliters of water into the reaction vessel, add 50 grams of bromoacetyl 7-ACA, add 23 milliliters of triethylamine dropwise at 2-5 ° C, after dissolving, add 20 grams of 4-mercaptopyridine, React at 5-10°C for 5 hours. After completion, add 150 milliliters of water, 5 grams of sodium thiosulfate, and 20 grams of sodium bicarbonate. After the solid dissolves, add 5 grams of activated carbon, decolorize for 30 minutes, filter the charcoal, and wash the charcoal layer with 20 milliliters of water. Add 35 grams of DBED to the filtrate, react at 37 to 40°C for 6 hours, filter, wash the product with 3×300 milliliters of water, and dry in vacuo to obtain white solid benzathine cefepirin. The molar yield is 94~97%, adopts HPLC method to record and contain cefapirin acid 80% (USP29 standard is 71.5~82.0%), adopts titration method to measure and c...
Embodiment 2
[0026] Embodiment 2: the preparation of benzathine cefapirin
[0027] Add 1003 milliliters of acetone, 783 milliliters of methanol and 391 milliliters of water into the reaction vessel, add 50 grams of bromoacetyl 7-ACA, add 23 milliliters of triethylamine dropwise at 2 to 5 ° C, after dissolving, add 14 grams of 4-mercaptopyridine, React at 5-10°C for 5 hours. After completion, add 150 milliliters of water, 5 grams of sodium thiosulfate, and 20 grams of sodium bicarbonate. After the solid dissolves, add 5 grams of activated carbon, decolorize for 30 minutes, filter the charcoal, and wash the charcoal layer with 20 milliliters of water. Add 35 grams of DBED to the filtrate, react at 37 to 40°C for 6 hours, filter, wash the product with 3×300 milliliters of water, and dry in vacuo to obtain a yellowish solid benzathine cefepirin. The molar yield was 60%.
Embodiment 3
[0028] Embodiment 3: the preparation of benzathine cefapirin
[0029] Add 1003 milliliters of acetone, 783 milliliters of methanol and 391 milliliters of water into the reaction vessel, add 50 grams of bromoacetyl 7-ACA, add 23 milliliters of triethylamine dropwise at 2-5 ° C, after dissolving, add 20 grams of 4-mercaptopyridine, React at 5-10°C for 2 hours. After completion, add 150 milliliters of water, 5 grams of sodium thiosulfate, and 20 grams of sodium bicarbonate. After the solid dissolves, add 5 grams of activated carbon, decolorize for 30 minutes, filter the charcoal, and wash the charcoal layer with 20 milliliters of water. Add 35 grams of DBED to the filtrate, react at 37~40° C. for 6 hours, filter, wash the product with 3×300 milliliters of water, and vacuum-dry to obtain white solid benzathine cefepirin, and the molar yield is 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com