Ph response type amphipathic stem-grafting polyphosphazenes feeding micelle and preparation method thereof
A polyphosphazene and responsive technology, which can be used in pharmaceutical formulations, antineoplastic drugs, drug combinations, etc., and can solve problems such as harsh conditions and medical application restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
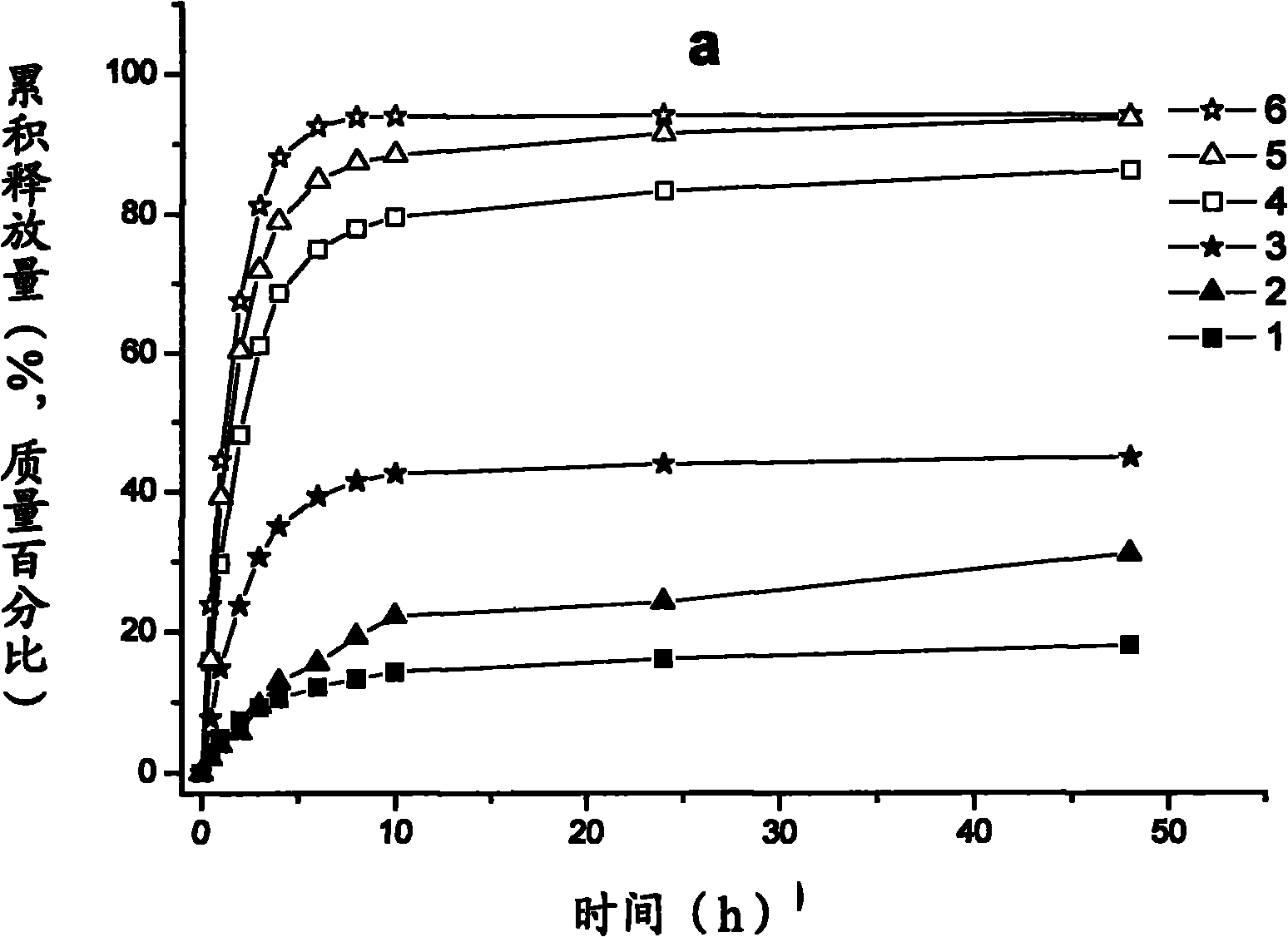
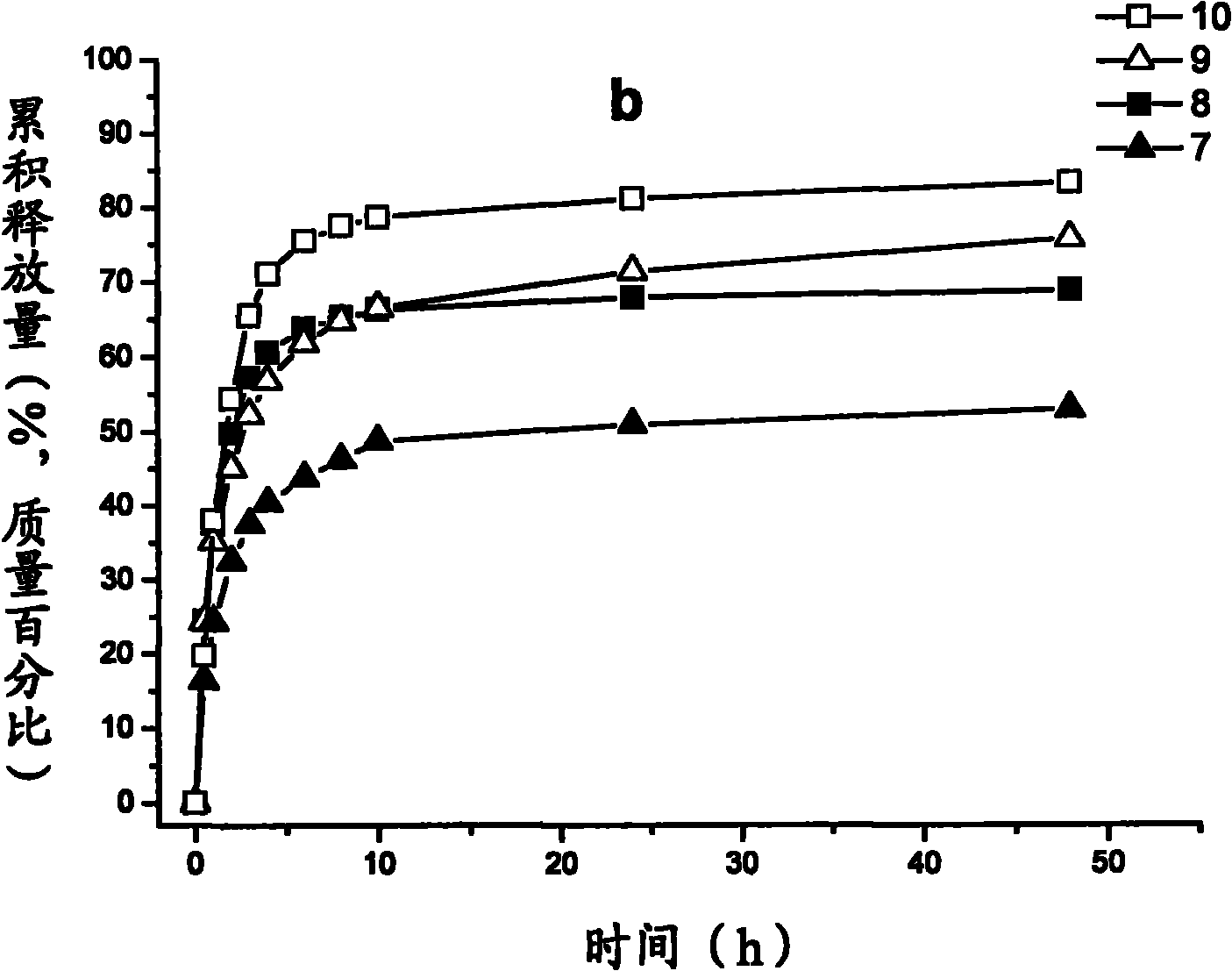
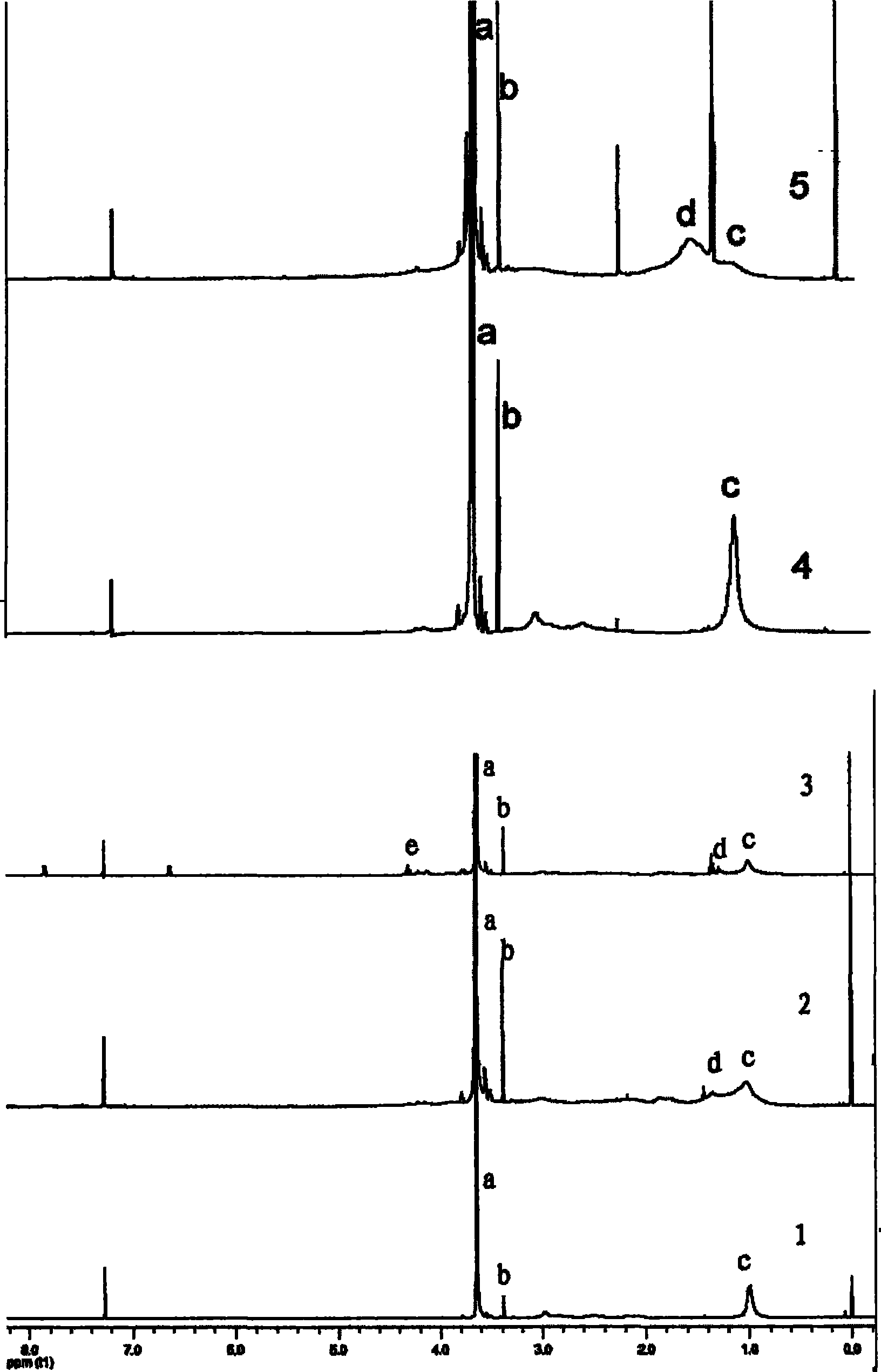
Image
Examples
Embodiment 1
[0088] Example 1 Synthesis method of pH-responsive amphiphilic grafted polyphosphazene
[0089] (1) Prepare poly(dichlorophosphazene) with aluminum chloride as catalyst
[0090] Weigh 4 g of chlorophosphazene cyclic trimer purified by sublimation and 0.2 g of anhydrous aluminum trichloride into a pre-cleaned and dried polymerization tube, vacuumize and seal the tube, carry out the polymerization reaction at 250°C for 5 hours, and wait for When the viscosity of the reactant remained almost constant, the polymerization was stopped and the polymerization tube was taken out to cool. Unseal the polymerization tube, add an appropriate amount of dry toluene solution to dissolve the reactants. After dissolution, it was precipitated with petroleum ether and dried in vacuum to obtain a white elastomer, that is, poly(dichlorophosphazene).
[0091] (2) Synthesis of pH-responsive amphiphilic grafted polyphosphazene copolymers via stepwise nucleophilic substitution reactions
[0092] Dis...
Embodiment 2
[0097] Example 2 Synthesis method of pH-responsive amphiphilic grafted polyphosphazene
[0098] (1) The preparation of poly(dichlorophosphazene) is the same as in Example 1.
[0099] (2) In addition to slowly adding 2.2g NH 2 - PEG (molecular weight is 2000) and 0.2ml TEA THF solution 50ml, containing 0.5g N,N-diisopropylethylenediamine (DPA) and 0.5ml TEA THF solution 10ml and containing 0.7g 4-aminobenzoic acid Except 10 milliliters of the THF solution of ethyl ester (EAB) and 0.5ml TEA, all the other operations are all the same as the step (2) in the embodiment 1, obtain the pH response type amphiphilic grafted polyphosphazene copolymer P2, in this copolymer The mass percent content of the amino-terminated polyethylene glycol of the hydrophilic segment is 60%, the mass percent content of N-[2-(N', N'-diisopropylamino) ethyl] amino group is 15%, and the N- The mass percent content of (ethyl benzoate)-4-amino is 20%, its number average molecular weight is 5000, molecular we...
Embodiment 3
[0103] Example 3 Synthesis method of pH-responsive amphiphilic grafted polyphosphazene
[0104] (1) The preparation of poly(dichlorophosphazene) is the same as in Example 1.
[0105] (2) In addition to slowly adding 6g NH 2 50ml of THF solution of PEG (molecular weight: 2000) and 0.6ml TEA, 10ml of THF solution containing 0.8g N,N-diisopropylethylenediamine (DPA) and 0.8ml TEA, 0.8g of 4-aminobenzoic acid 10 ml THF solution of ethyl ester (EAB) and 0.7ml TEA and 10 ml THF solution of 2.0g β-hydroxyalanine ethyl ester (SEE) and 1.5ml TEA, and react at room temperature after each dropwise addition of a class of raw materials Except for 8h, all the other operations are the same as step (2) in Example 1 to obtain the pH-responsive amphiphilic grafted polyphosphazene copolymer P3, the mass percentage of the hydrophilic segment amino-terminated polyethylene glycol in the copolymer Content is 70%, the mass percent content of N-[2-(N ', N'-diisopropylamino) ethyl] amino is 10%, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com