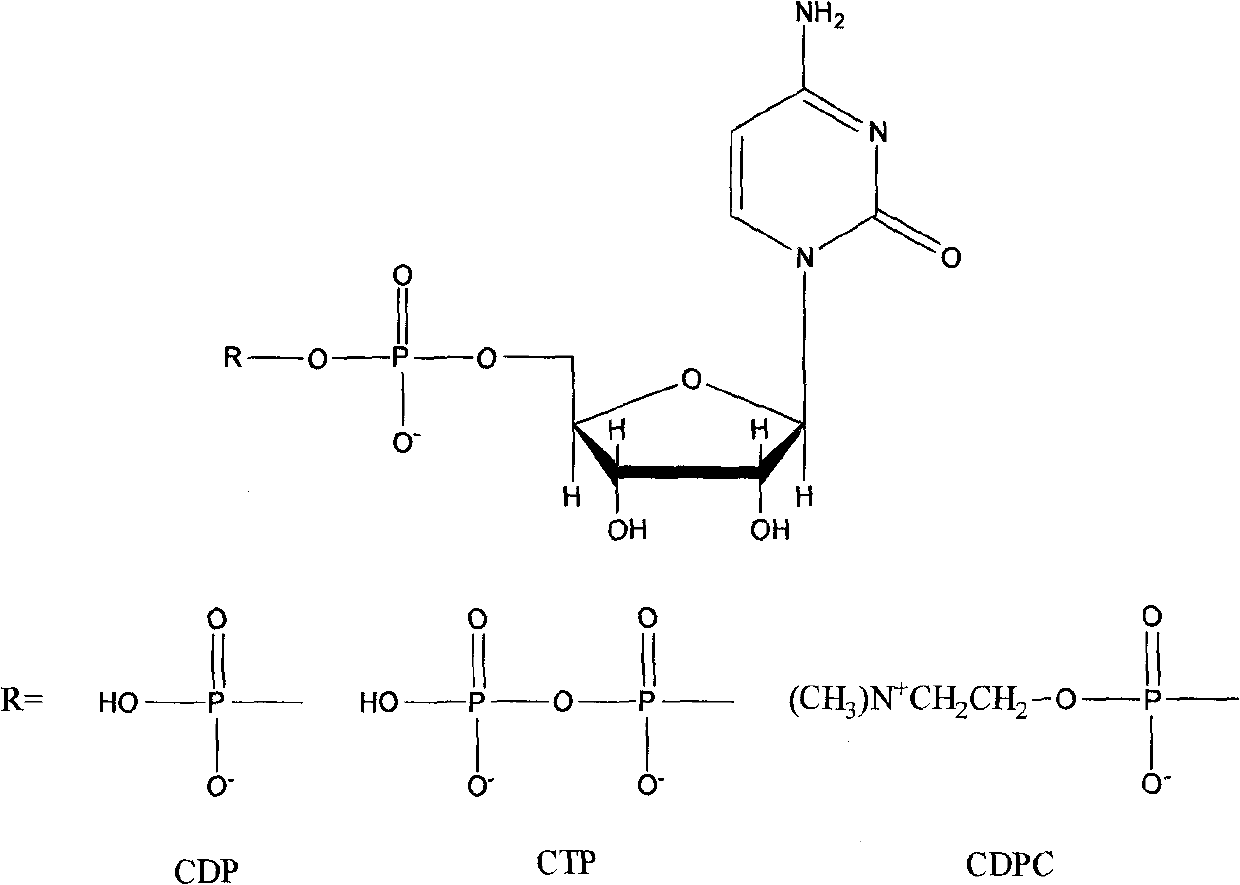
Method for synthesizing cytidine phosphinylidyne compounds through oriented catalysis
A cytidine phosphoryl compound and substrate technology, which is applied in the field of biocatalysis, can solve the problems of conversion rate, low product concentration, complex cell enzyme system, long fermentation period and the like, and achieves low production cost, simple reaction system and convenient production. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Yeast medium: glucose 40g / L, urea 2.0g / L, potassium dihydrogen phosphate 1.5g / L, zinc sulfate heptahydrate 4.0×10 -3 g / L, ferrous sulfate heptahydrate 3.0×10 -3 g / L, manganese chloride tetrahydrate 0.3×10 -3 g / L, anhydrous calcium chloride 1.0×10 -3 g / L, biotin 0.05×10 -3 g / L.
[0038] The inoculum amount of yeast was 10%, cultured on a shaker at 120 rpm at 30°C for 24 hours, and centrifuged at 4000 rpm for 20 minutes. Take the yeast paste and store it at -7°C for later use.
Embodiment 2
[0039] Example 2: Directional production of CTP by CMP.
[0040] Prepare in the reaction tank of capacity 15L by CMP 300mmol, choline chloride 100mmol, glucose 5mol, manganese sulfate 500mmol, dithiothreitol 300mmol, Bacillus subtilis 2400g cultivated by the method for embodiment 1, potassium dihydrogen phosphate 3mol , Triton X-1001g and 10L of reaction solution composed of water, adjust the pH to 7.0 with sodium hydroxide, and the temperature is 35°C, finish the reaction after 3 hours of reaction, precipitate with perchloric acid, and quantitatively analyze the product by HPLC, The main product in the conversion liquid is CDP, its content is 18.6mmol / L, and the yield is 62.1%. At this time, the content of CDP is 2.5mmol / L, and the content of CDPC is 5.8mmol / L.
Embodiment 3
[0041] Example 3: Directional production of CTP by CMP.
[0042] Prepare in the reaction tank of capacity 15L by CMP 300mmol, choline chloride 3000mmol, glucose 5mol, manganese nitrate 500mmol, dithiothreitol 300mmol, brewing yeast 2500g cultivated by the method of embodiment 1, potassium dihydrogen phosphate 3mol, 10L of reaction solution composed of 10mL of toluene and water was adjusted to pH 7.0 with sodium hydroxide and the temperature was 40°C. After 3 hours of reaction, the reaction was terminated, precipitated with perchloric acid, and the product was quantitatively analyzed by HPLC. The main product in the conversion solution was It is CDP, its content is 27.5mmol / L, and the yield is 91.8%. At this time, the CDP content is 0.7mmol / L, and the CDPC content is 0.8mmol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com