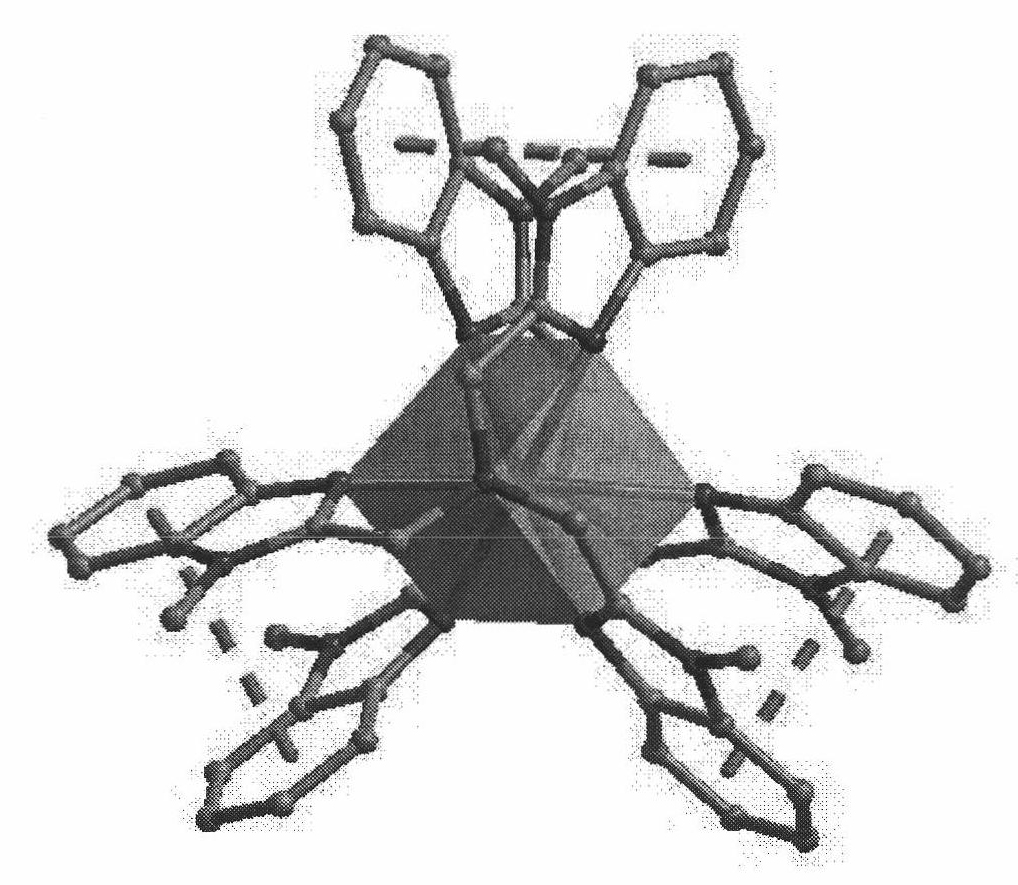
Gasochromic rare earth complex and preparation method and application thereof
A rare-earth complex and aerochromic technology, which are applied to compounds containing group 3/13 elements of the periodic table, color-changing fluorescent materials, chemical instruments and methods, etc., can solve the problems of luminous brightness and monochromaticity, response efficiency, and stability The complexity of preparation of active complexes is low, the performance parameters cannot meet the needs of aerochromic materials, and the production and promotion of materials are restricted, so as to achieve the effects of improved response intensity, high yield, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Preparation of ligand:
[0026] (1) Add 16.2g (0.15mol) of o-phenylenediamine and 9.6g (0.05mol) of amine triacetic acid into a 250ml round bottom flask, add 60ml of ethylene glycol and stir evenly, heat the oil bath to 140-200 degrees to reflux 8-12 hours, during the reaction process, a water separator can be installed to draw the generated water out of the reaction system; after the reaction, cool slightly while stirring and pour into 200ml of water, filter, recrystallize ethanol (decolorize with activated carbon if necessary), and obtain the product NTB (Yield 85%).
[0027] (2) In a 150ml three-necked flask, 1.02g (0.0025mol) NTB and 50ml of tetrahydrofuran treated with sodium metal were added in batches under the protection of nitrogen flow, 0.3g (0.0077mol, due to the oxidation of potassium and the incomplete removal of Water, metal potassium requires a little excess) metal potassium until no hydrogen is produced, then refluxed for 1-2 hours, and the system be...
Embodiment 2
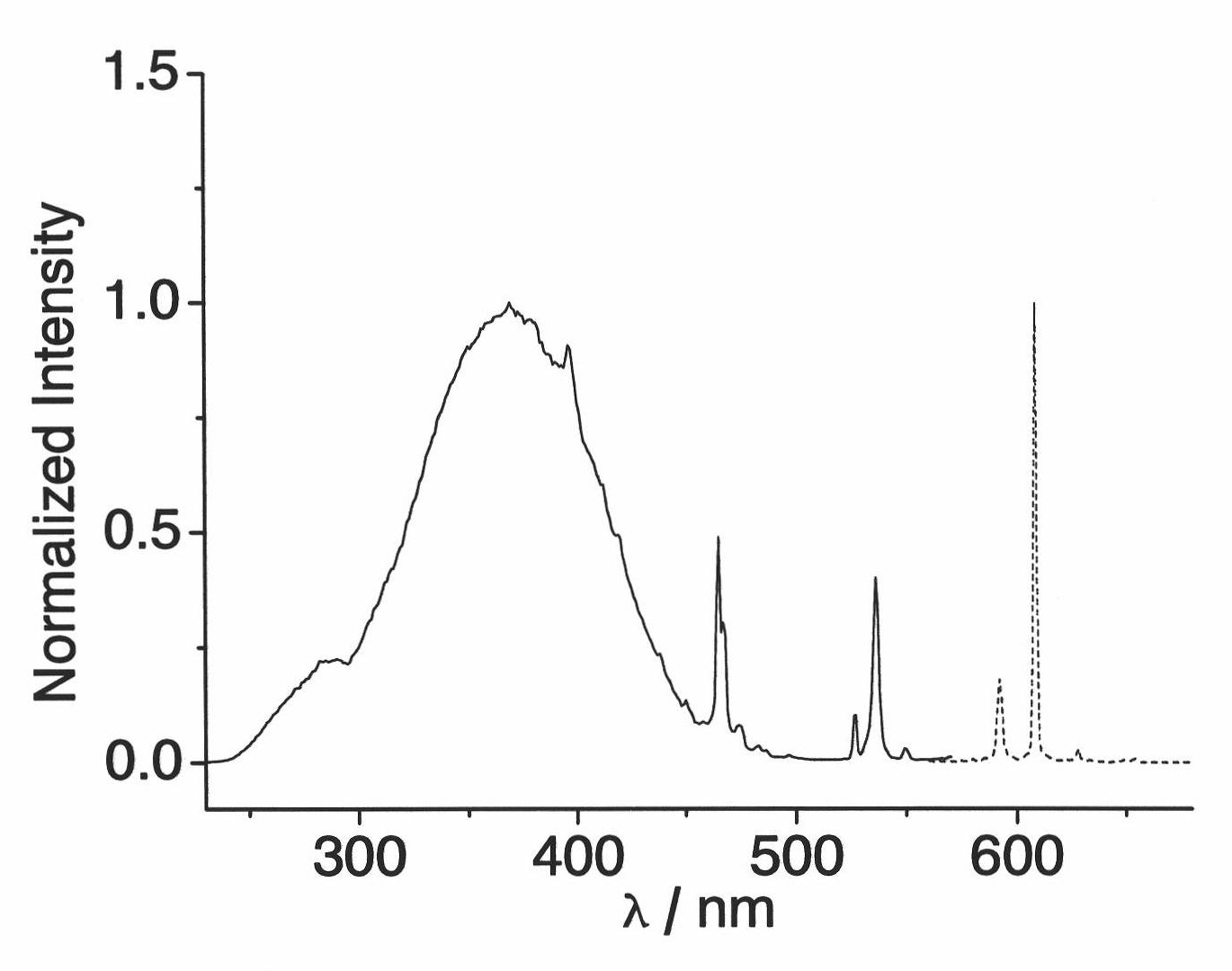
[0032] The test of the luminescent properties of the complex: measured by the FLS920 fluorescence analyzer of EDINBURGH Company in the United Kingdom, the sample emits the characteristic linear emission of Eu(III) ions at 594, 611, and 620 nm under the excitation of 370 nm incident light.
[0033] The fluorescence test shows that under the excitation of 370nm ultraviolet light, the ligand absorbs energy and transfers it to the rare earth metal, emitting the characteristic red line emission of Eu(III), as shown in the attached figure 2 shown.
Embodiment 3
[0035] Test of gasochromism of complexes:
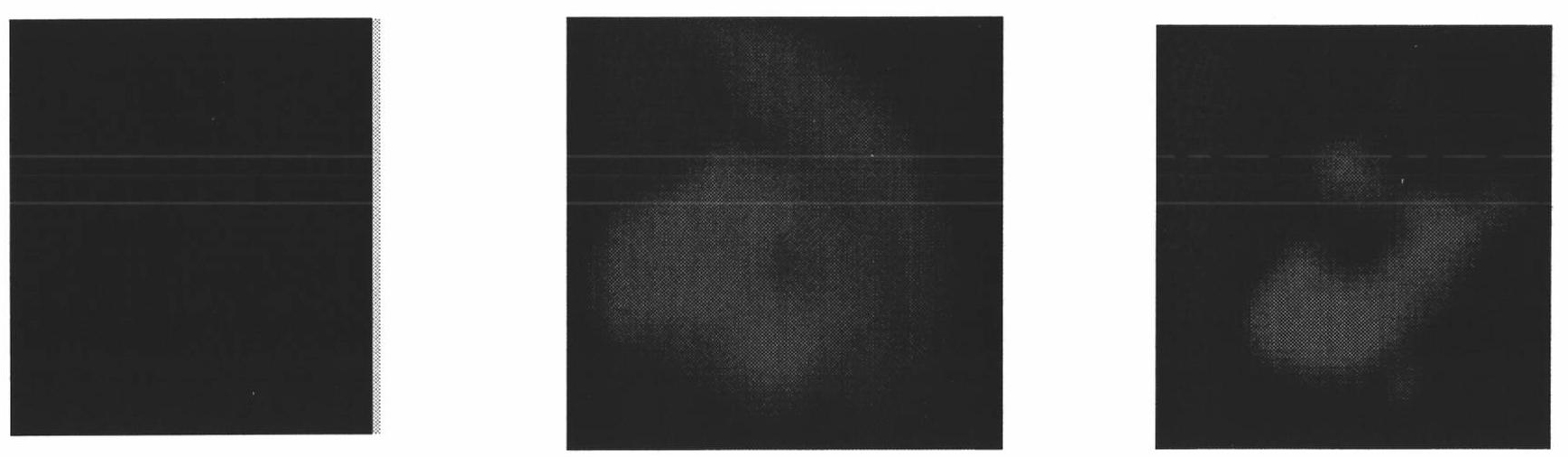
[0036] The complex was exposed to methanol or acetonitrile vapor for 10 minutes, and the schematic diagram of the fluorescence enhancement under the vapor atmosphere is shown in the attached image 3 shown. Pictured (left) is the initial sample, (middle) exposed to an atmosphere of methanol, and (right) exposed to an atmosphere of acetonitrile.
[0037] The results show that when the complex is exposed to methanol or acetonitrile vapor, obvious aerochromic phenomenon occurs, and the fluorescence intensity is increased by 18.1 times and 15 times respectively, and the response speed is fast. Reach the strongest within.
[0038] Then methanol or acetonitrile vapor was removed, and nitrogen gas was introduced for 10 minutes. The fluorescence intensity of the former weakened and returned to the initial state before the methanol vapor was not passed, while the fluorescence intensity of the latter did not weaken with the removal of aceton...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com