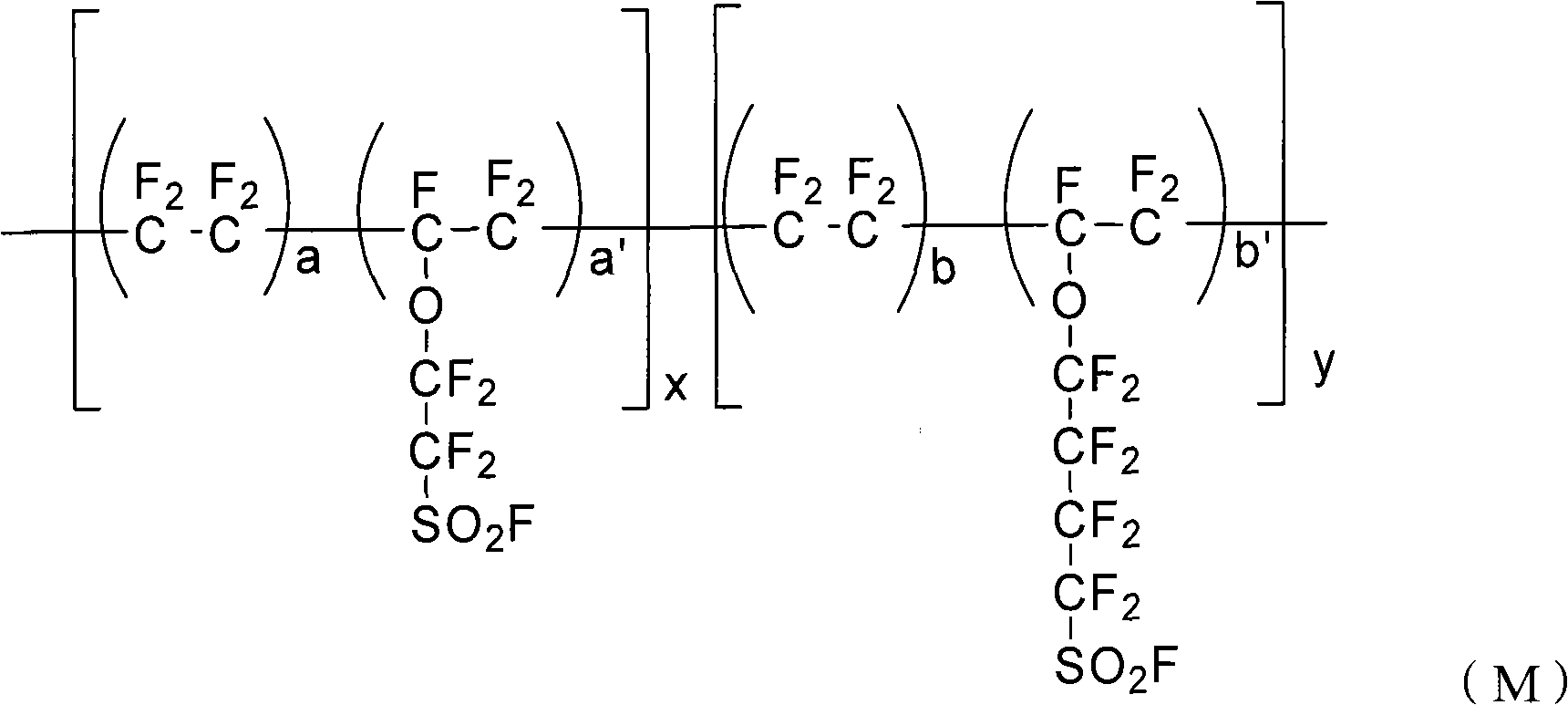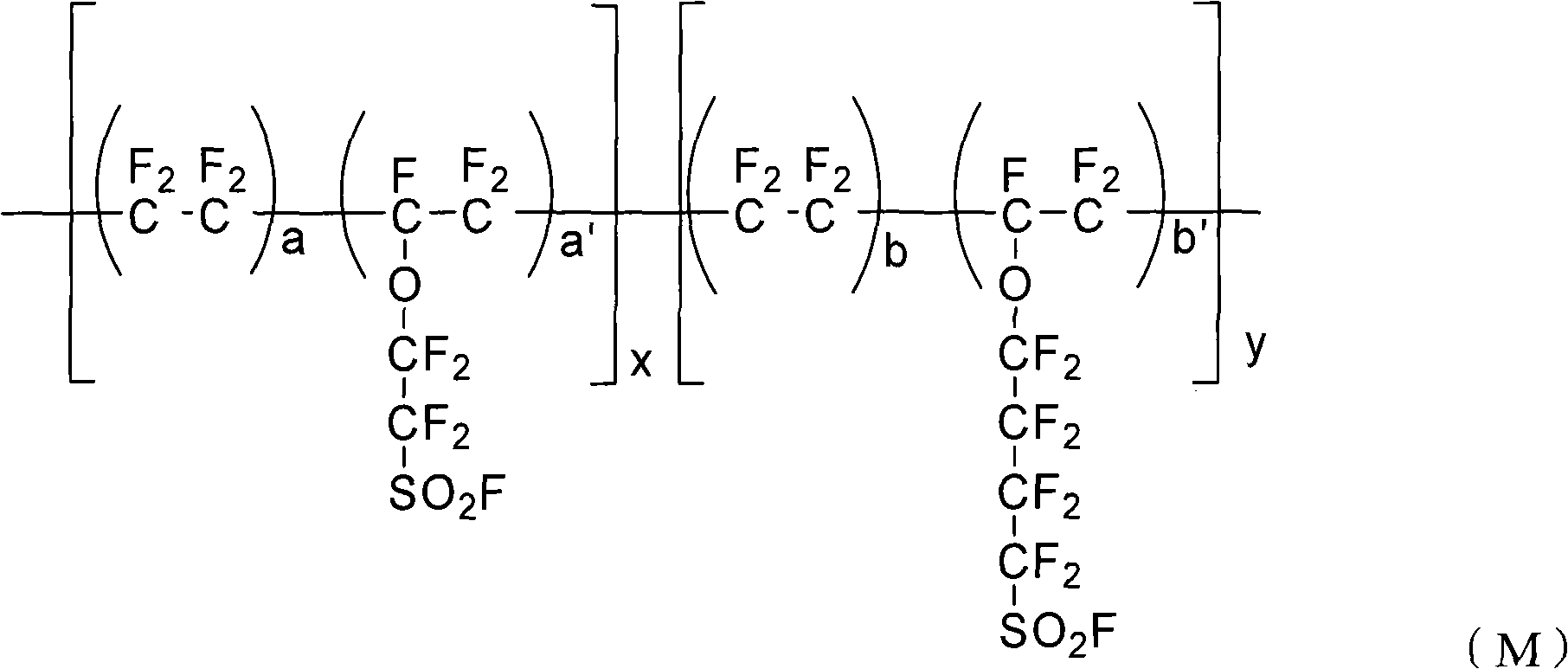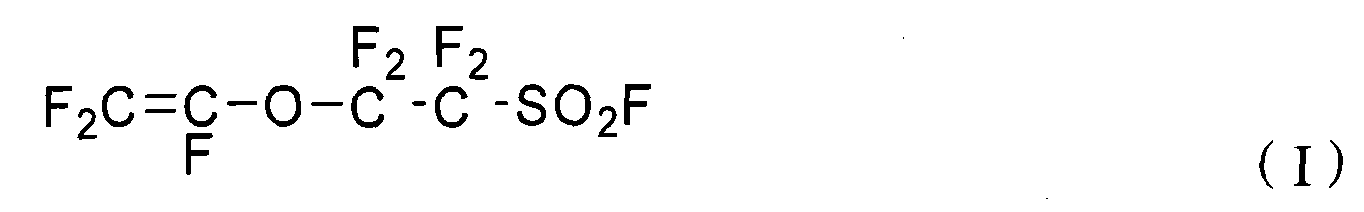Perfluorinated ion exchange resin and preparation method and application thereof
An exchange resin and perfluorinated ion technology, applied in cation exchange materials, structural parts, battery pack parts, etc., can solve the problems of poor thermal stability and the inability of perfluorinated ion exchange resin to meet the mechanical strength and ion exchange capacity at the same time. Achieve the effects of good thermal stability, good high temperature mechanical stability, and high ion exchange capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] This example is used to illustrate the preparation process and measurement results of the perfluorinated ion exchange resin provided by the present invention.
[0033] making process:
[0034] (a) Clean the reactor and add 5L deionized water and 200g sodium dodecylbenzenesulfonate, start the stirring device, vacuumize and fill with high-purity nitrogen for three times, after testing the oxygen content in the reactor is below 1ppm, Vacuumize, add 400g sulfonyl fluoride pendant vinyl ether monomer (I) (F 2 C=CF-O-CF 2 -CF 2 -SO 2 F) and 550g sulfonyl fluoride pendant vinyl ether monomer (II) (F 2 C=CF-O-CF 2 -CF 2 -CF 2 CF 2 -SO 2 F);
[0035] (b) Fill tetrafluoroethylene monomer (CF 2 = CF 2 ) to a pressure of 2.5MPa;
[0036] (c) The reaction kettle is heated to 50° C., and 3.2 g of perfluorobutyryl peroxide (CF 3 CF 2 CF 2 CO-OO-CCF 2 CF 2 CF 3 ) to initiate the polymerization reaction, continue to feed tetrafluoroethylene monomer to keep the reactio...
Embodiment 2
[0041] This example is used to illustrate the preparation process and measurement results of the perfluorinated ion exchange resin provided by the present invention.
[0042] making process:
[0043] (a) Wash the reactor and add 5L deionized water, 150g sodium dodecylbenzenesulfonate and 125g nonylphenol polyoxyethylene ether NP-10, start the stirring device, vacuumize and fill with high-purity nitrogen for three times, After the oxygen content in the test reactor is below 1ppm, vacuumize, and add 500g of sulfonyl fluoride pendant vinyl ether monomer (I) (F) to the reactor through the liquid feed valve. 2 C=CF-O-CF 2 -CF 2 -SO 2 F) and 400g sulfonyl fluoride pendant vinyl ether monomer (II) (F 2 C=CF-O-CF 2 -CF 2 -CF 2 CF 2 -SO 2 F);
[0044] (b) Fill tetrafluoroethylene monomer (CF 2 = CF 2 ) to a pressure of 5.5 MPa;
[0045] (c) The reactor is warmed up to 35°C, and 8g perfluoropropoxypropyl peroxide (CF) is added to the kettle by a metering pump. 3 CF 2 CF ...
Embodiment 3
[0050] This example is used to illustrate the preparation process and measurement results of the perfluorinated ion exchange resin provided by the present invention.
[0051] making process:
[0052] (a) Clean the reactor and add 5L of deionized water and 500ml of alkyl ether sulfate Texapon NSOIS with a solid content of 27%, start the stirring device, vacuumize and replace with high-purity nitrogen for three times, and test the oxygen content in the reactor After below 1ppm, vacuumize, add 300g sulfonyl fluoride pendant vinyl ether monomer (I) (F 2 C=CF-O-CF 2 -CF 2 -SO 2 F) and 610g sulfonyl fluoride pendant vinyl ether monomer (II) (F 2 C=CF-O-CF 2 -CF 2 -CF 2 CF 2 -SO 2 F);
[0053] (b) Fill tetrafluoroethylene monomer (CF 2 = CF 2 ) to a pressure of 3.2MPa;
[0054] (c) The reaction kettle is heated to 80° C., and 320 g of 10% by weight of ammonium persulfate aqueous solution is added to the kettle by a metering pump to initiate a polymerization reaction, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com