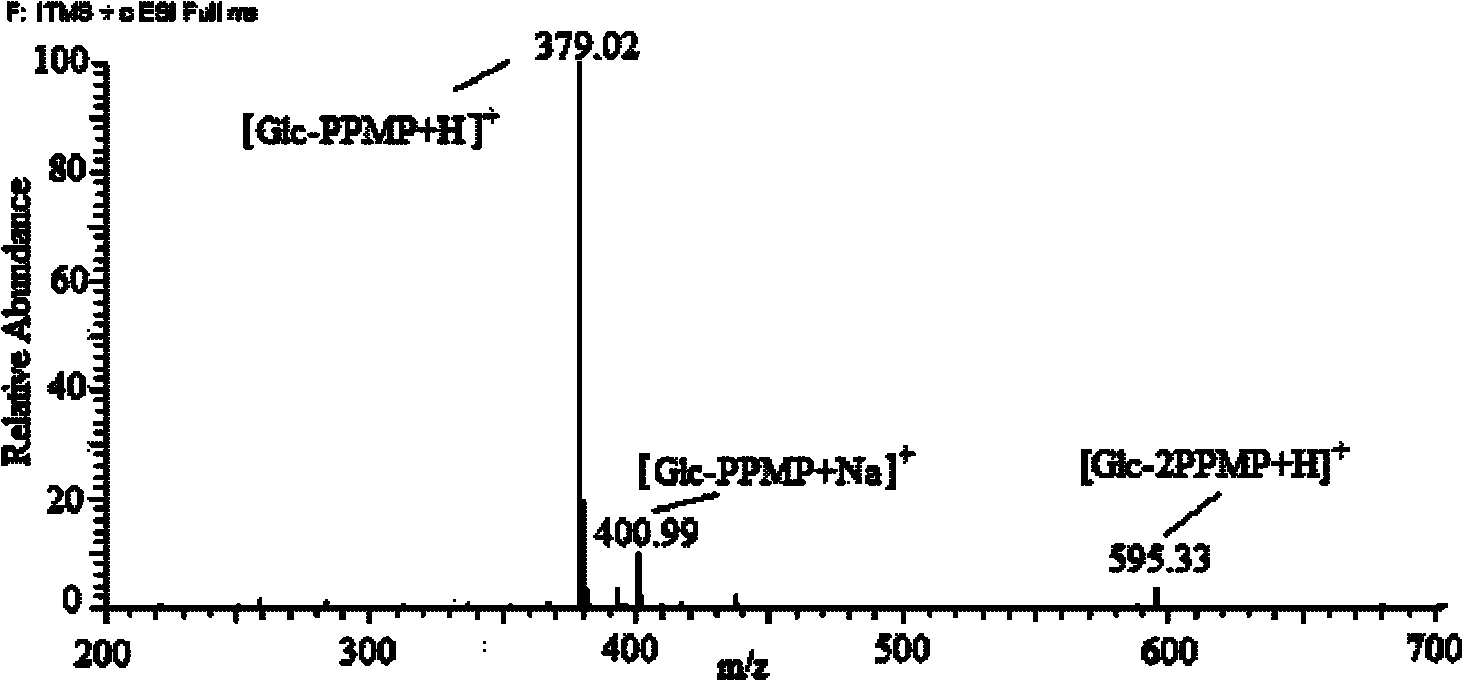
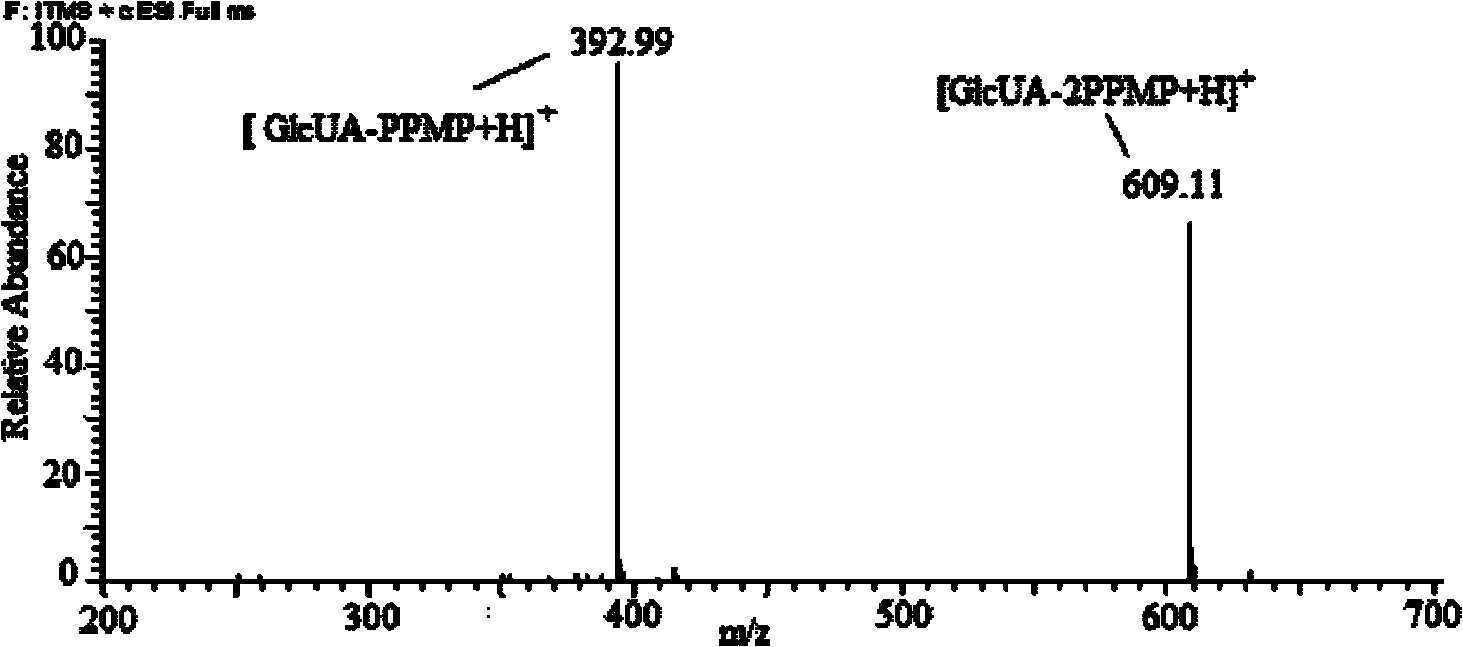
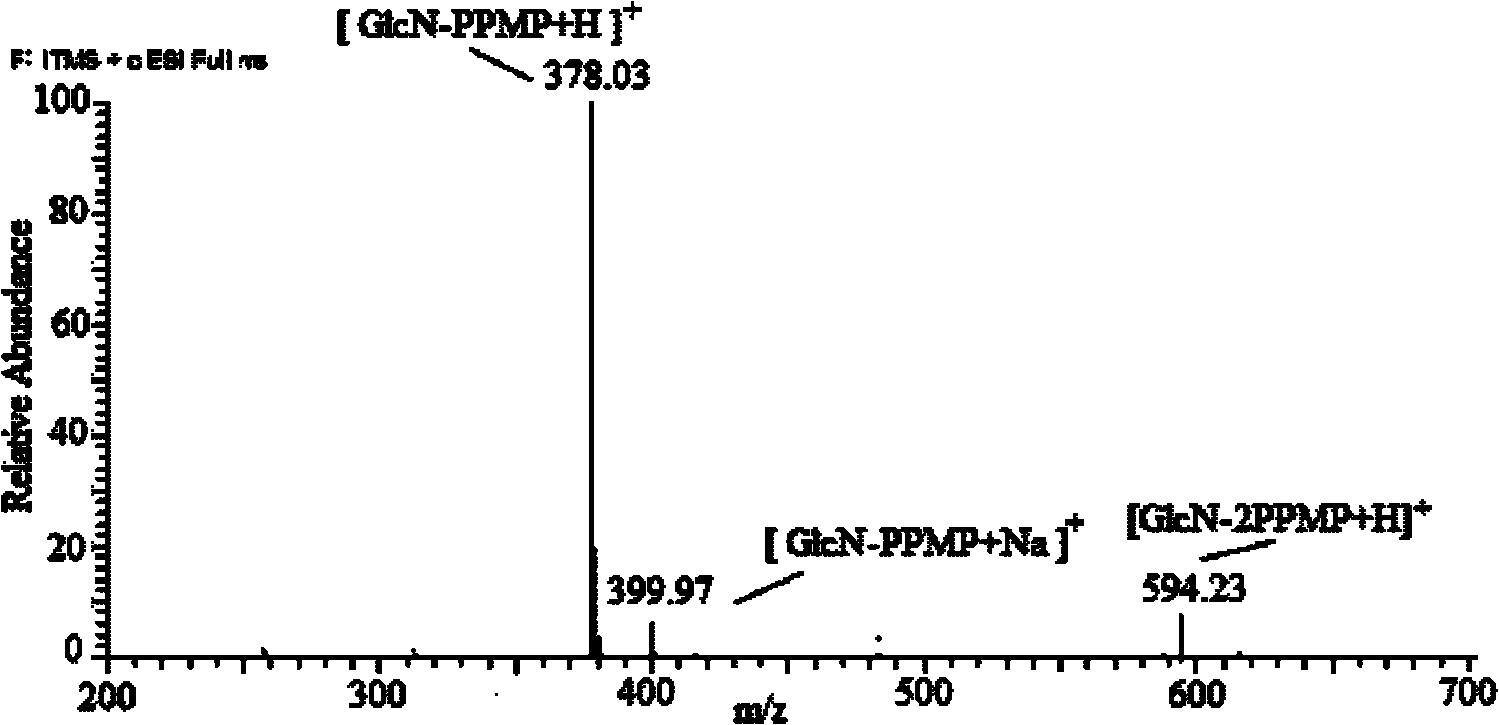
1-(4-isopropylbenzene-yl)-3-methyl-5-pyrazolone, preparation method and application thereof
A technology of cumylphenyl and pyrazolone, applied in the field of organic chemical synthesis, can solve the problems of cumbersome removal of excess reagents, few types of separated monosaccharides, high polarity, etc., and achieve mild conditions, less environmental pollution, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the synthesis of 1-phenyl-3-methyl-5-pyrazolone
[0019] Weigh 2.160 g (0.02 mol) of phenylhydrazine, add 2.5 mL of ethyl acetoacetate and 2.5 mL of glacial acetic acid dropwise, and react with magnetic stirring for about 4 hours at room temperature, and detect the end point of the reaction by TLC. The glacial acetic acid was evaporated in vacuum, the product was dissolved in methanol, recrystallized, filtered, and the filter cake was air-dried to constant weight to obtain 2.701 g of off-white powder 1-phenyl-3-methyl-5-pyrazolone (PMP) , and the yield was 77.58%.
Embodiment 2
[0020] Embodiment 2: Synthesis of 1-(4-isopropylphenyl)-3-methyl-5-pyrazolone
[0021] Weigh 3.734 g (0.02 mol) of 4-isopropylphenylhydrazine hydrochloride, add dropwise 17 mL of 5% NaOH solution for neutralization, and at room temperature, stir and react for 4 h, then neutralize with 1 mol / L hydrochloric acid to pH= 8-9, then use CH 2 Cl 2 After extraction and evaporation to dryness, 2.90 g (0.0192 mol) of light yellow solid 4-isopropylphenylhydrazine was obtained, with a yield of 96.76%.
[0022] To the solid 4-isopropylphenylhydrazine obtained above, 2.5 mL of ethyl acetoacetate and 2.9 mL of glacial acetic acid were added dropwise, and the reaction was carried out under magnetic stirring for about 4 hours at room temperature, and the end point of the reaction was detected by TLC. The glacial acetic acid was evaporated in vacuo, the product was passed through a silica gel column, and eluted with petroleum ether: ethyl acetate = 4:1; collected by TLC, and a small amount of...
Embodiment 3
[0023] Embodiment 3: Synthesis of 1,3-diphenyl-5-pyrazolone
[0024] Weigh 2.160g (0.02mol) of phenylhydrazine, add 3.46mL of ethyl benzoylacetate and 2.5mL of glacial acetic acid dropwise, and grind and stir at room temperature for about 3 hours to generate a large amount of white milky solid. The end point of the reaction is detected by TLC. The solid product was washed with a small amount of n-hexane, recrystallized with methanol-ethanol, filtered, and the filter cake was naturally dried to constant weight to obtain 4.418 g of milky white powder 1,3-diphenyl-5-pyrazolone (DPP for short). The yield was 93.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com