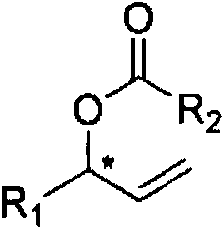
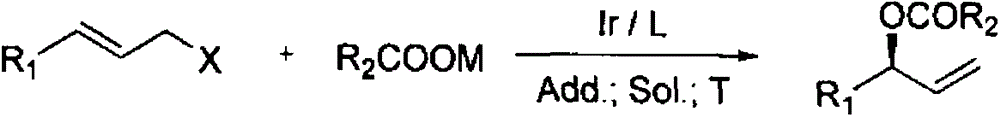Chiral allyl ester compound and preparation method thereof
A technology for compounds and allyl esters, applied in the field of organic chemical synthesis, can solve the problems of low optical purity of target products, difficulty in obtaining enantioselectivity, low yield of target products, etc., and achieves a wide range of substrates and easy-to-obtain catalysts. , the effect of good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In a dry reaction tube protected by argon, 0.002 mmol of dibenzo-1,5-cyclooctadiene iridium chloride dimer, 0.004 mmol of chiral ligand L, 1,4-diazabicyclo [2.2.2] Octane 0.002mmol and dioxane 1mL were reacted at 30°C for 0.2h to prepare an iridium catalyst.
[0047] Add 0.2 mmol of potassium acetate to the catalytic system, then add 0.22 mmol of allyl halide, 0.2 mmol of KCl, and 2.0 mL of THF, and stir at room temperature for 6 hours. After the reaction is completed, pass through a diatomite sand core and remove the solvent under reduced pressure. Chromatography yielded the target product 1 (petroleum ether / ethyl acetate=40 / 1).
[0048] Target product 1: (S)-1-phenylallyl ethyl ester
[0049]
[0050] Yellow liquid, 91% yield, 94%ee [chiral column OJ-H (0.46cm x 25cm); n-hexane / isopropanol=95 / 5; flow rate=0.6mL / min; detection wavelength=214nm; t R =21.012(minor), 23.24l(major)min].
[0051] HRMS (ESI + )calcd for C 11 h 12 NaO 2 [M+Na] + : 199.0735, Found: ...
Embodiment 2
[0053] In a dry reaction tube protected by argon, add 1,5-cyclooctadiene (H 5 -indene)iridium 0.001mmol, chiral ligand L 0.002mmol, isopropylamine 0.002mmol and tetrahydrofuran 1mL, react at 40°C for 0.4h.
[0054] Add 0.2mmol of potassium acetate to the catalytic system, then add 0.4mmol of allyl halide, 0.2mmol of CsCl, and 2.0mL of diethyl ether, stir at room temperature for 2h, after the reaction is completed, filter, and then distill to remove low boilers to obtain the target product 2 .
[0055] Target product 2: (S)-1-p-methoxyphenyl allyl ethyl ester
[0056]
[0057] Yellow liquid, 92% yield, 95%ee [chiral column OJ-H (0.46cm x 25cm); n-hexane / isopropanol=95 / 5; flow rate=0.6mL / min; detection wavelength=214nm; t R = 18.12 (minor), 19.24 (major) min].
[0058] HRMS (ESI + )calcd for C 11 h 12 NaO 2 [M+Na] + : 229.0841, Found: 229.0844.
Embodiment 3
[0060] In a dry reaction tube protected by argon, 0.0015 mmol of 1,5-cyclooctadiene iridium chloride dimer, 0.003 mmol of chiral ligand L, 1,8-diazabicyclo[5.4. 0] Undec-7-ene 0.003mmol and THF 1.5mL were reacted at 80°C for 0.5h.
[0061] Add 0.2mmol of sodium acetate to the catalytic system, then add 0.4mmol of allyl halide, 0.2mmol of CsF, and 2.0mL of acetonitrile, and stir at 120°C for 2h. The target product 3 was obtained by recrystallization of cyclohexane at 5°C.
[0062] Target product 3: (S)-1-p-methylphenylallyl ethyl ester
[0063]
[0064] Colorless liquid, 93% yield, 91%ee [chiral column OJ-H (0.46cm x 25cm); n-hexane / isopropanol=95 / 5; flow rate=0.6mL / min: detection wavelength=214nm; 1 R = 11.52 (minor), 12.93 (major) min].
[0065] HRMS (ESI + )calcd for C 11 h 12 NaO 2 [M+Na] + : 213.0891, Found: 213.0864.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com