Iridium complex containing boron mesityl unit, preparation method and application as fluorescent probe
A technology of iridium complex and mi-based boron is applied in the field of cationic iridium complex phosphorescent materials to achieve the effect of improving selectivity, high selectivity, and avoiding the interference of system luminescence background and scattering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, when with for for When, the preparation of the ligand:
[0023] Compound 1: Preparation of 5,5'-dibromo-2,2'-bipyridine
[0024] Take by weighing 2,5-dibromopyridine (2.0g, 8.7mmol) and tetrakis (triphenylphosphorous palladium) (0.2g, 0.2mmol) and join in the two-necked bottle, and take light-proof measure to reaction device, then in Vacuum-fill nitrogen-vacuumize the double-row tube, cycle three times, and then protect the reaction system with nitrogen. P-xylene (70 mL) which had been distilled and deoxygenated by nitrogen bubbling was injected into the reaction system with a syringe, and then hexa-n-butyldistannane (2.46 mL) was injected. The reaction temperature was raised to 130° C., and the reaction was stirred for 3 days. After the reaction of the raw materials is complete, the reaction temperature is lowered to room temperature, and then the reaction mixture is poured into a saturated aqueous solution of EDTA (about 250 mL), and stirred ...
Embodiment 2
[0031] Embodiment 2, when with for for When, the preparation of the complex:
[0032] Weigh IrCl 3 ·3H 2 O (1mmol) and C^N ligand Bpq (2.5mmol) were added into a three-necked flask, and the reaction system was protected with nitrogen. Inject the mixture of 2-ethoxyethanol and water (3:1v / v) into the reaction system with a syringe, stir, and raise the temperature of the reaction system to 110°C. The reaction time is about 24 hours. During the reaction, precipitates are formed. . The reaction system was cooled to room temperature, then the precipitate was filtered, and washed with water and ethanol to obtain a red solid product, i.e. iridium dichloro bridge compound (Bpq) 2 Ir(μ-Cl) 2 Ir(Bpq) 2 . Weigh iridium dichloro bridge compound (Bpq) 2 Ir(μ-Cl) 2 Ir(Bpq) 2 (0.2mmol) and oligomeric N^N ligand (0.5mmol) and join in the there-necked flask, protect the reaction system with nitrogen. A mixture of dichloromethane and methanol (2:1, v / v) was injected into the...
Embodiment 3
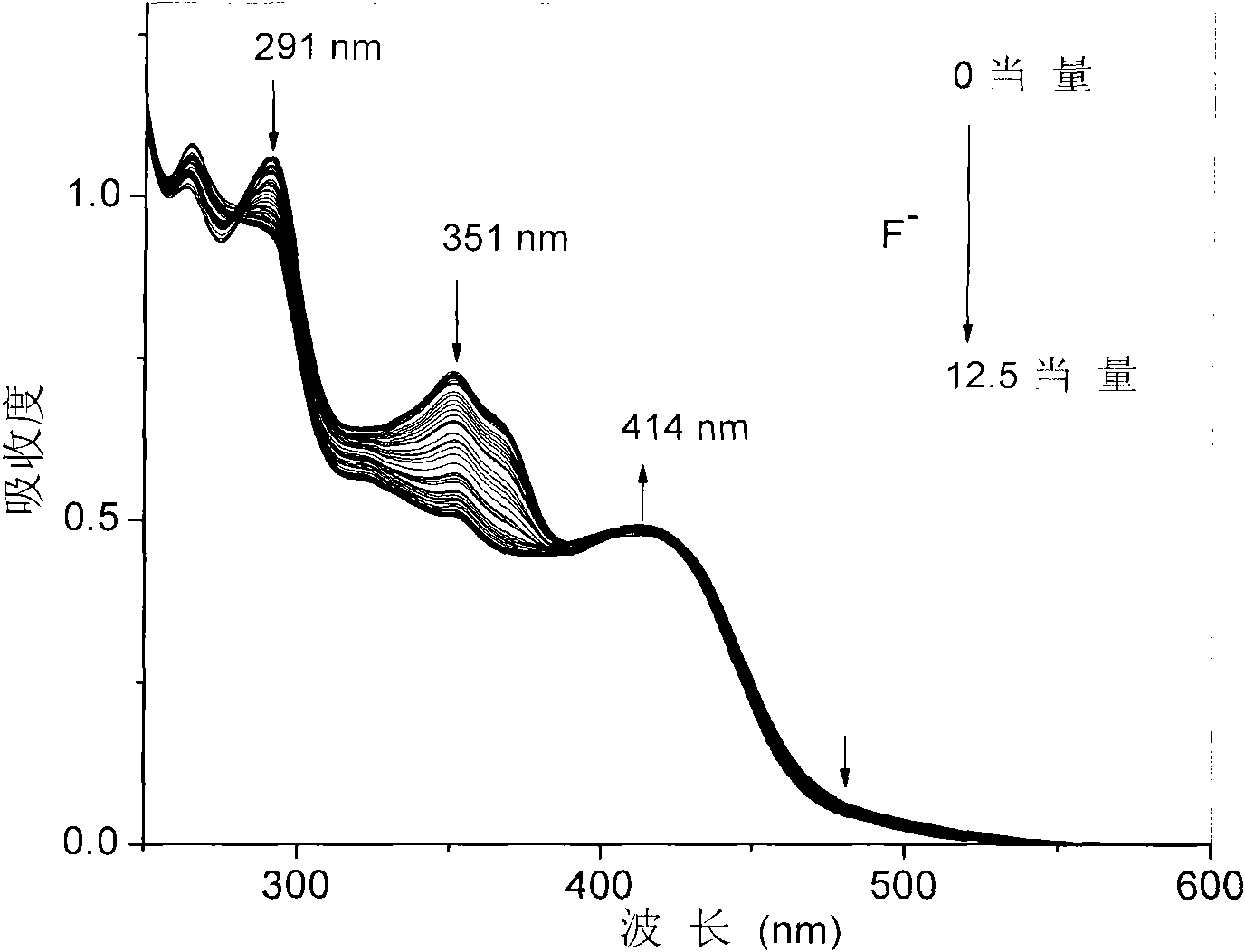
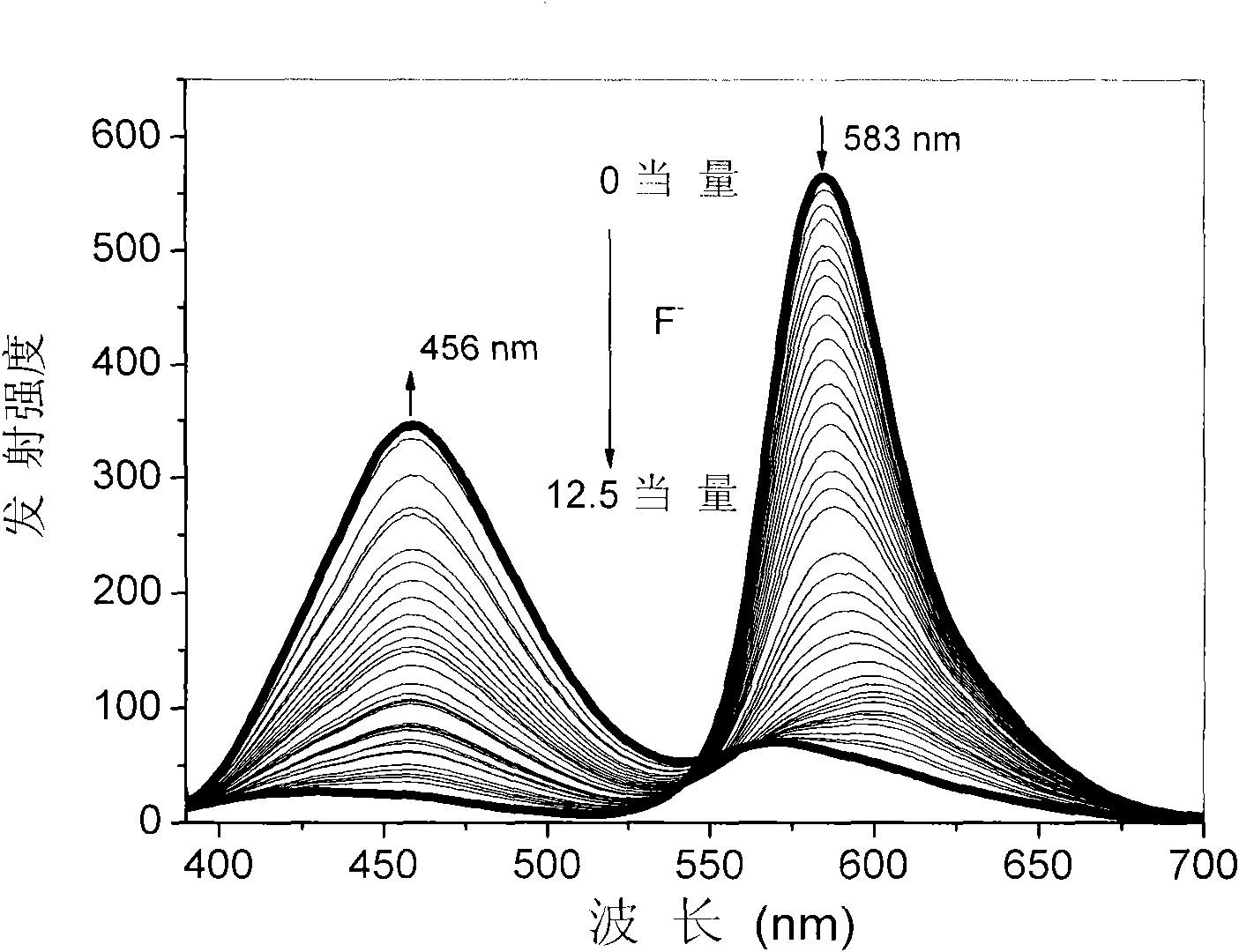
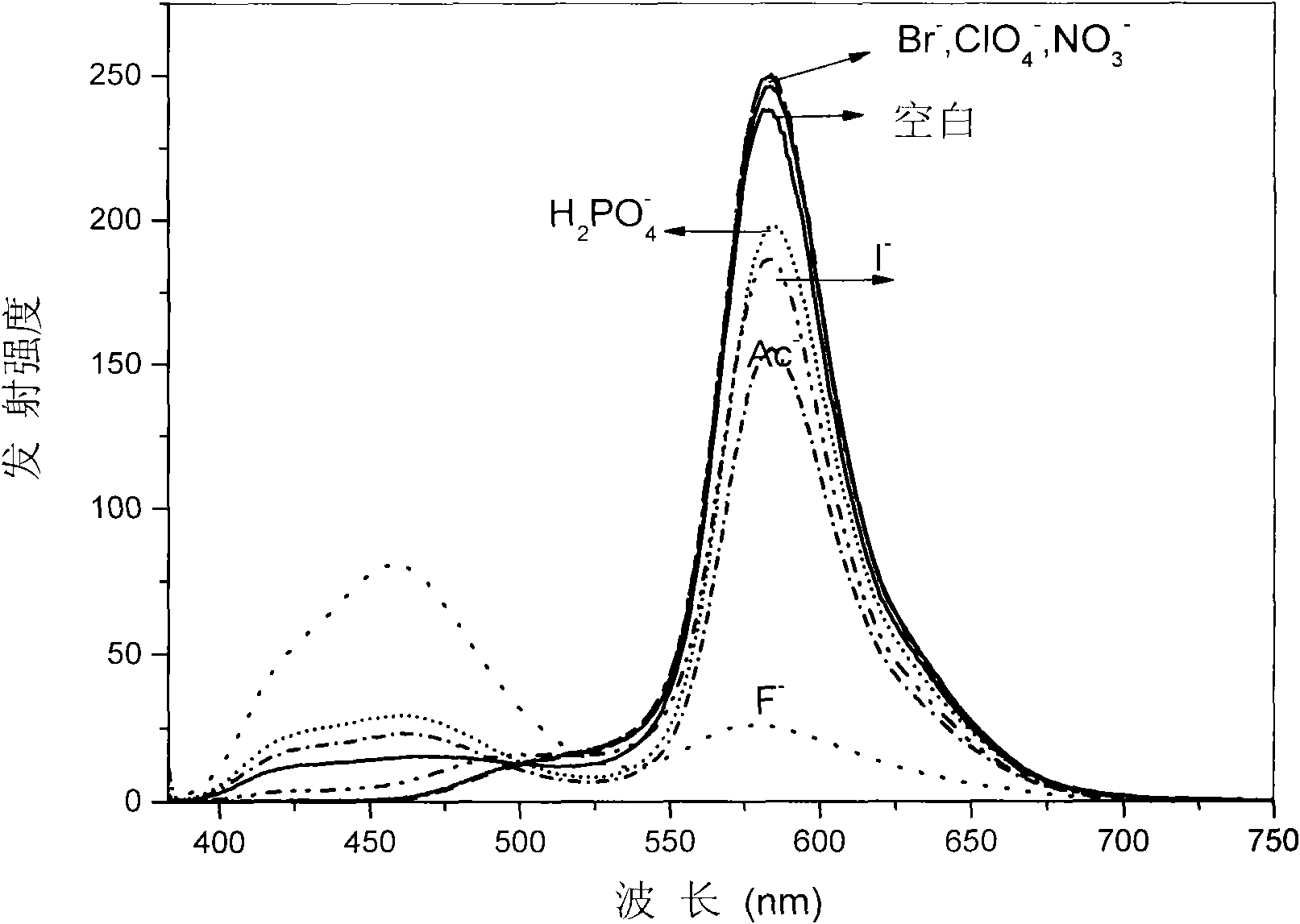
[0033] Embodiment 3, to F in the solution - Titration experiment
[0034] Prepare 20μM complex solution (THF or dichloromethane as solvent), pipette 2.5mL complex solution into a fluorescence cuvette, gradually add dropwise 2.5×10 -3 mol / LF-solution (acetonitrile as solvent) until reaching equilibrium (that is, the spectrum no longer changes significantly), respectively measured without adding F - and drop different contents of F - UV-Vis and photoluminescence spectra, such as figure 1 with figure 2 shown. Test data show that: with F - The orange-red light emission based on the main chain iridium complex gradually weakens, and the blue light emission based on the oligomeric N^N ligand CzbpyCz gradually increases, and the emission color changes from orange-red to blue.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com