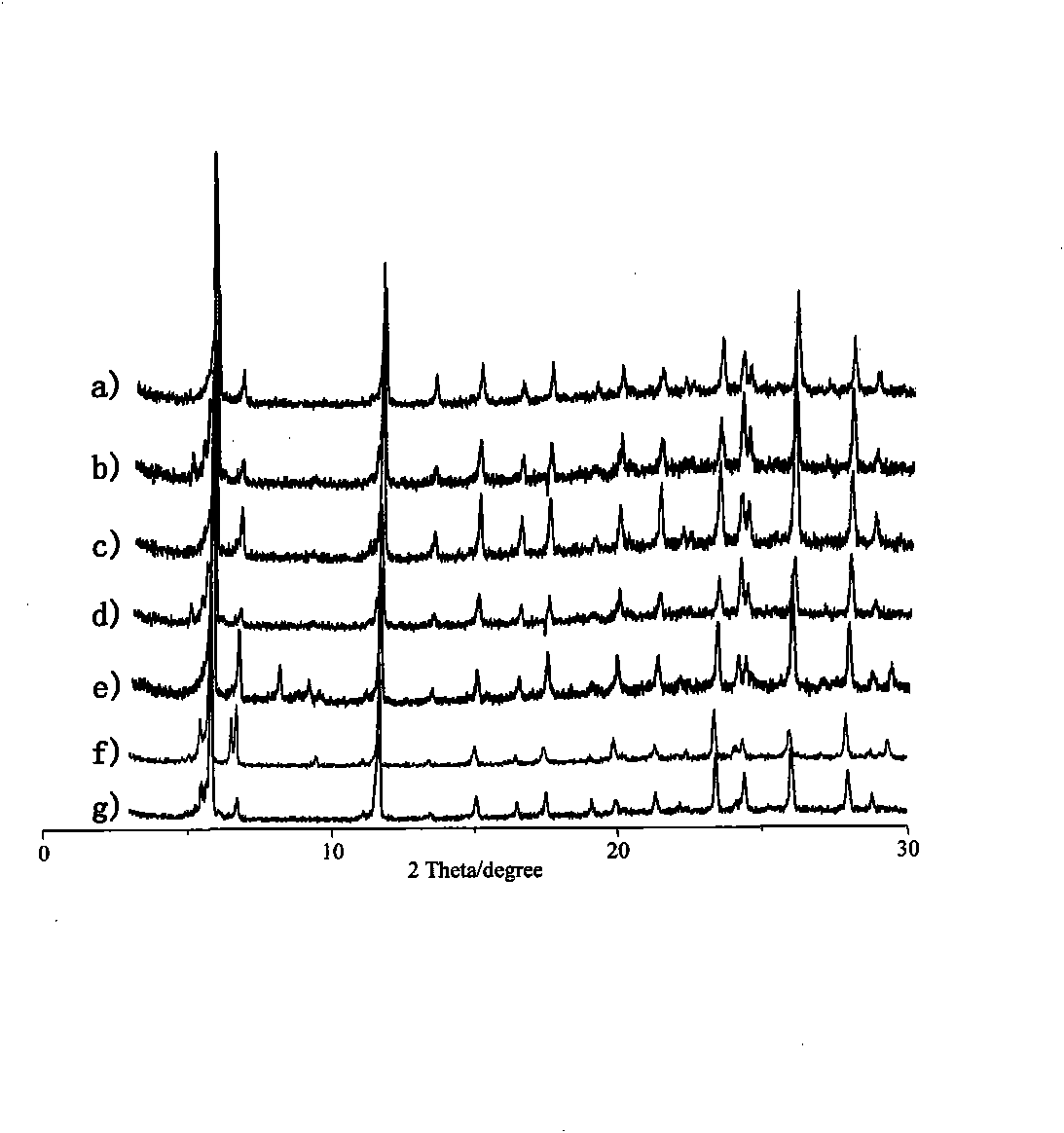
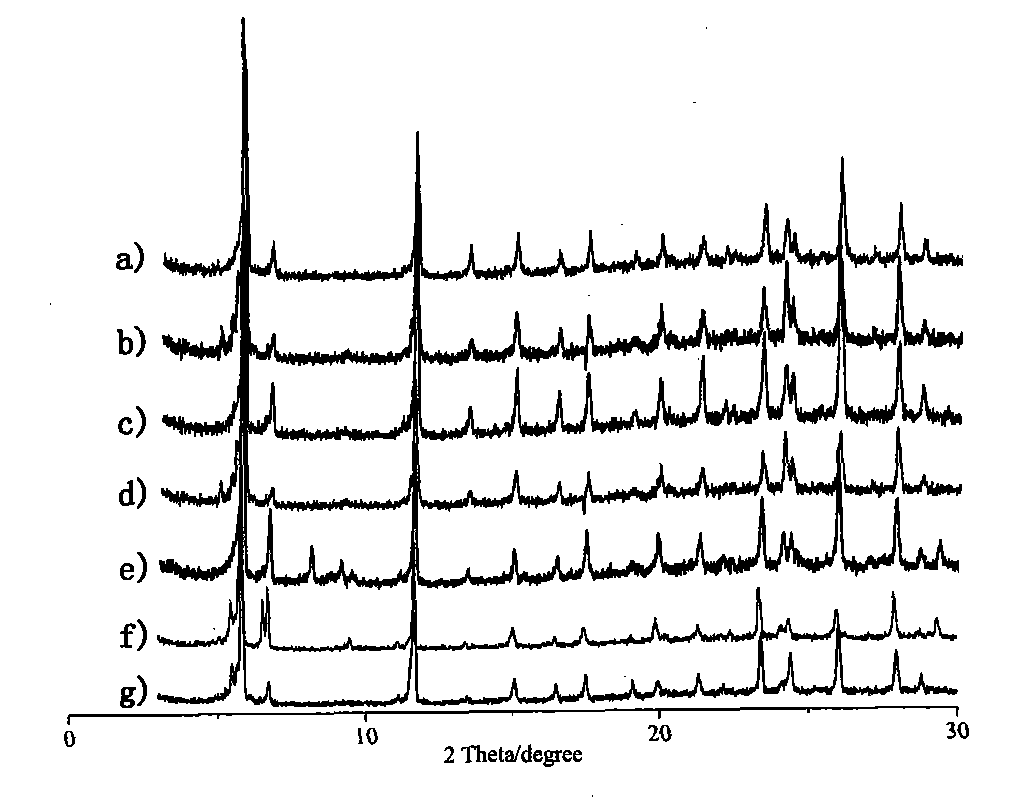
Crystalline catalysis material for reaction of preparing dimethyl ether from methanol by dehydration and preparation method thereof
A catalytic material, methanol dehydration technology, applied in the field of chemical materials, can solve the problems of high molecular sieve cost, high reaction temperature, non-uniform active points, etc., and achieve the effect of high catalytic active point density, reduced reaction cost, and high water resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: [Cu 2 (BTC) 4 / 3 ] 6 [H 4 SiW 12 o 40 ] preparation
[0026] H was prepared according to the literature method 4 SiW 12 o 40 ·xH 2 O. (North, E.O.Inorg.Synth.1939, 1, 129.) H 4 SiW 12 o 40 ·xH 2 O, Trimellitic acid, copper sulfate, tetramethylammonium chloride and water are mixed at a material ratio of 1:5:10:8:2000, stirred, and the pH of the mixture is adjusted to 0.5, under hydrothermal autogenous pressure conditions Under 120 degrees Celsius, crystals were obtained after 8 hours. The crystals were collected, refluxed in ethanol, and heated to 160°C under vacuum, and the target crystalline material was obtained after 2 hours.
Embodiment 2
[0027] Example 2: [Cu2 (BTC) 4 / 3 ] 6 [H 4 GeW 12 o 40 ] preparation
[0028] H was prepared according to the literature method 4 GeW 12 o 40 14H 2 O. (Rocchiccioli-Deltcheff et al.Inorg.Chem.1983, 22, 207-216) H 4 GeW 12 o 40 14H 2 O, Trimellitic acid, copper chloride, tetraethylammonium chloride and water are mixed at a material ratio of 1: 22: 30: 25: 24000, stirred, and the pH of the mixture is adjusted to 2.5. Under the condition of 240 degrees Celsius, crystals were obtained after 48 hours. The crystals were collected, refluxed in ethanol, and heated to 240°C under vacuum, and the target crystalline material was obtained after 12 hours.
Embodiment 3
[0029] Embodiment 3: [Cu 2 (BTC) 4 / 3 ] 6 [H 3 PMo 12 o 40 ] preparation
[0030] H was prepared according to the literature method 3 PMo 12 o 40 14H 2 O. (Rocchiccioli-Deltcheff et al.Inorg.Chem.1983, 22, 207-216) H 3 PMo 12 o 40 14H 2 O, Trimellitic acid, copper nitrate, ammonium chloride and water are mixed at a material ratio of 1:5:10:8:2000, stirred, and the pH of the mixture is adjusted to 0.5. Under hydrothermal autogenous pressure conditions, 120 degrees Celsius, crystals were obtained after 8 hours. The crystals were collected, refluxed in ethanol, and heated to 160°C under vacuum, and the target crystalline material was obtained after 2 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com