Method for producing superfine aluminium hydroxide and aluminium oxide by using flyash
A technology of ultra-fine aluminum hydroxide and fly ash, applied in alumina/aluminum hydroxide, products, reagents, etc., can solve the problems of complicated production process, increase production cost, etc., achieve high selectivity, reduce emissions, The effect of reducing silicon content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
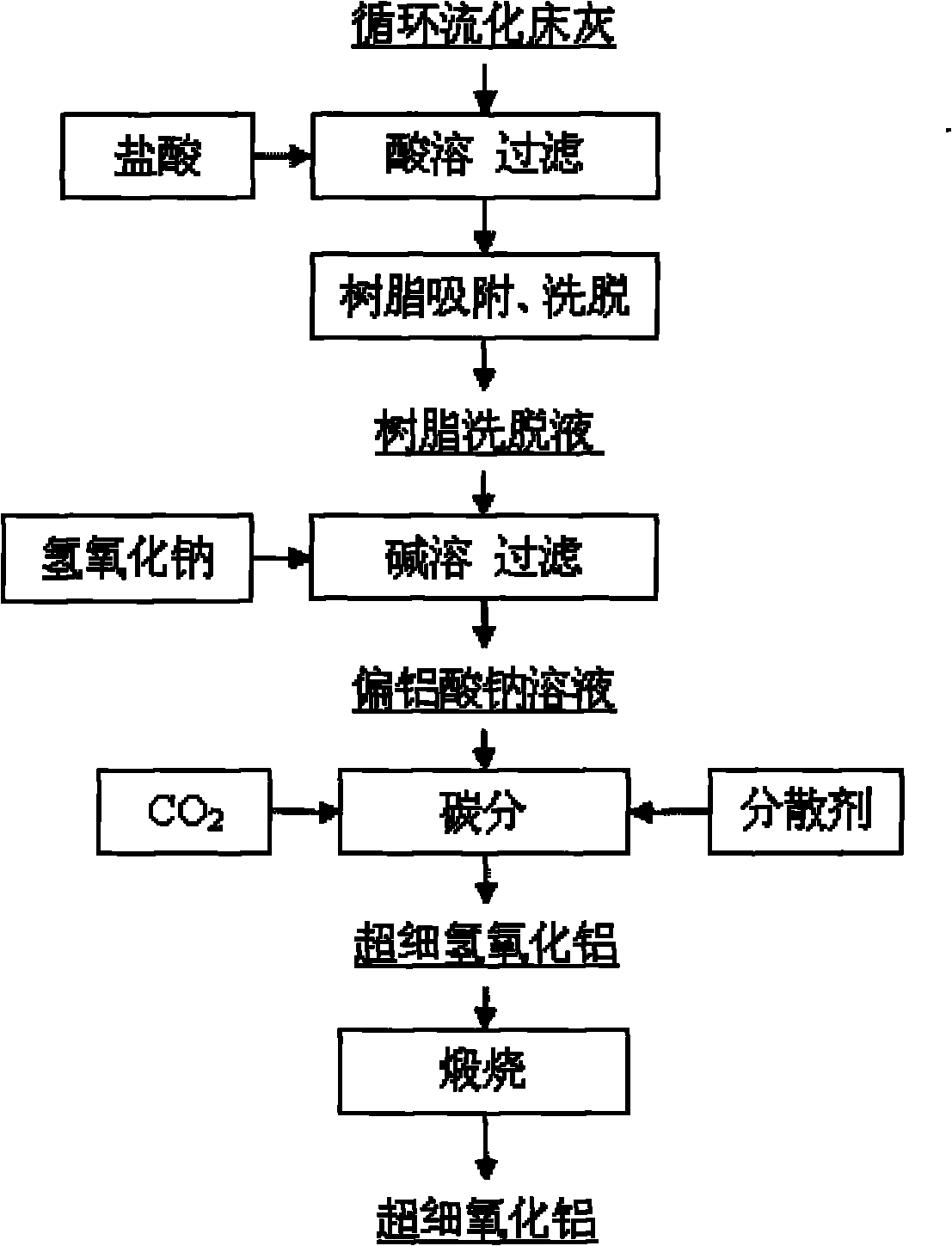
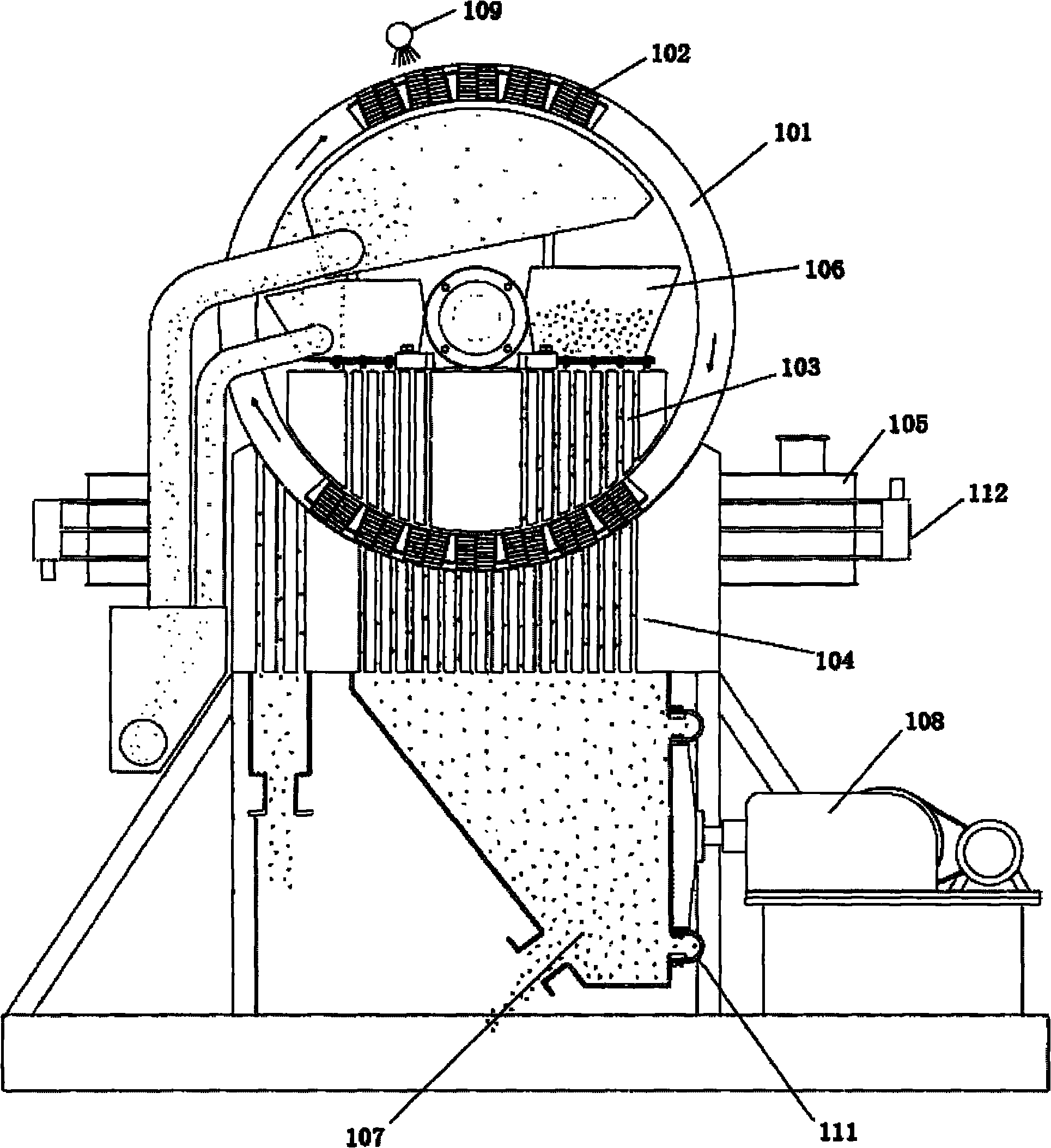
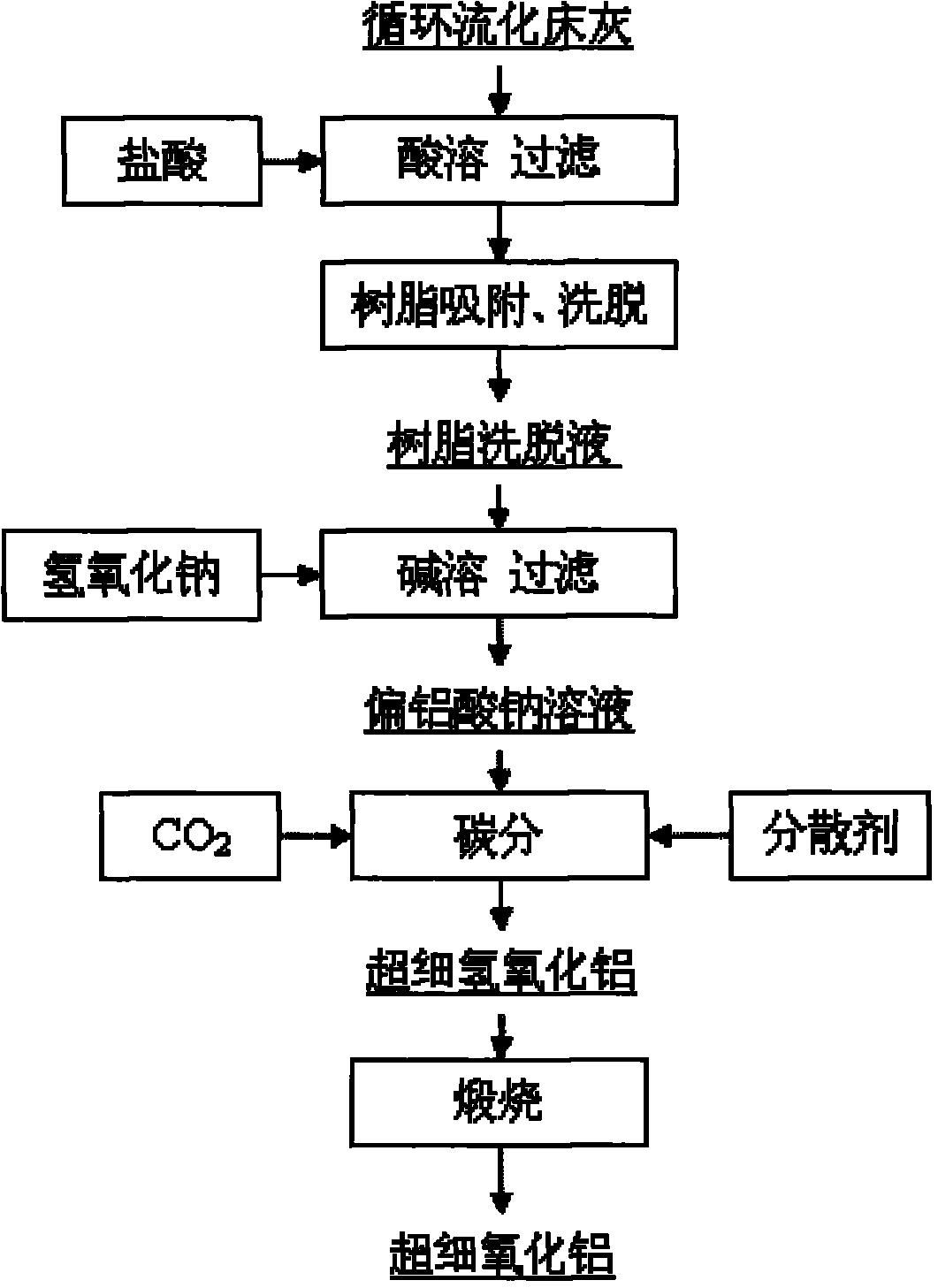
[0046] (1) Get circulating fluidized bed fly ash, pulverize to 200 orders, use embodiment 12 to be used for the vertical ring magnetic separator of fly ash iron removal to carry out wet magnetic separation and make the iron oxide content in the ash down to 0.8wt %. The filter cake after the magnetic separation is put into an acid-resistant reaction kettle, and the industrial hydrochloric acid with a concentration of 37wt% is added to carry out acid-dissolving reaction. The molar ratio of HCl in hydrochloric acid to alumina in fly ash is 4.5:1, and the reaction temperature is 200°C. Pressure 2.1MPa, reaction time 1 hour. After the reaction product was filtered and washed by a plate basket filter press, a hydrochloric acid immersion solution with a pH value of 1.7 was obtained. It was determined that the extraction rate of alumina in the fly ash was 84.2%.
[0047] (2) After cooling the hydrochloric acid immersion solution to 65°C through heat exchange, use a corrosion-resistan...
Embodiment 2
[0053] Except step (1), other operating process conditions are all identical with embodiment 1. The operating process condition adjustment in the step (1) is:
[0054] (1) Get circulating fluidized bed fly ash, pulverize to 150 orders, use embodiment 12 to be used for the vertical ring magnetic separator of fly ash to remove iron to carry out wet magnetic separation and make the iron oxide content in the ash drop to 0.8wt %. The filter cake after the magnetic separation is put into an acid-resistant reaction kettle, and the industrial hydrochloric acid with a concentration of 28wt% is added to carry out acid-dissolution reaction. The molar ratio of HCl in the hydrochloric acid and alumina in the fly ash is 5: 1, and the reaction temperature is 150°C. Pressure 1.0MPa, reaction time 2 hours. The reaction product was filtered and washed by a plate basket filter press to obtain a hydrochloric acid immersion solution with a pH value of 1.5. It was determined that the extraction r...
Embodiment 3
[0057] Except step (1), other operating process conditions are all identical with embodiment 1. The operating process condition adjustment in the step (1) is:
[0058] (1) Get circulating fluidized bed fly ash, pulverize to 200 orders, use embodiment 12 to be used for the vertical ring magnetic separator of fly ash iron removal to carry out wet magnetic separation and make the iron oxide content in the ash down to 0.8wt %. The filter cake after the magnetic separation is put into an acid-resistant reaction kettle, and the industrial hydrochloric acid with a concentration of 20wt% is added to carry out the acid-dissolving reaction. The pressure is 0.1MPa, and the reaction time is 4 hours. After the reaction product was filtered and washed by a basket filter press, a hydrochloric acid immersion solution with a pH value of 1.4 was obtained, and the extraction rate of alumina in the fly ash was determined to be 80.1%.
[0059] After determination, the chemical composition and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com