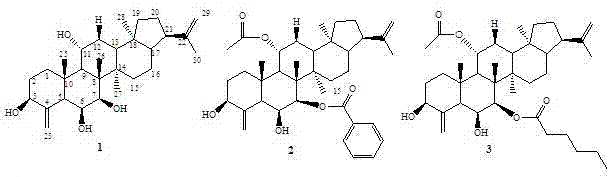
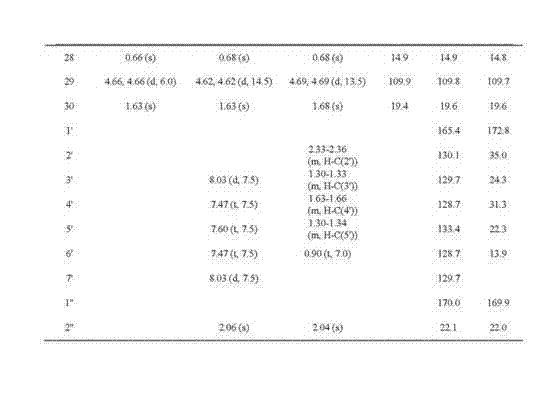
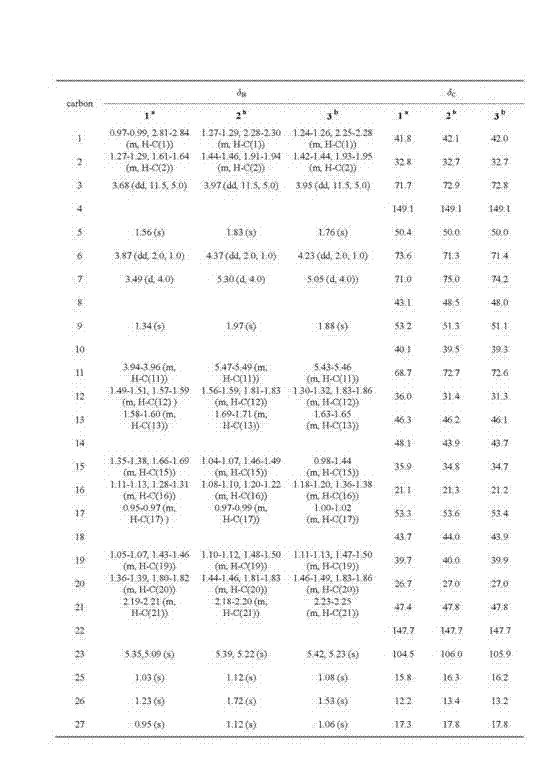
New hopane-type triterpene and preparation method and application thereof
A technology of Hobane type and petroleum ether, which is applied in the field of medicine and can solve problems such as unseen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) 15 kg of dry twigs of Cymbidium spp. were extracted three times by reflux of 95% ethanol, each time for 2 hours, and the extract was recovered under reduced pressure to obtain crude ethanol extract;
[0018] (2) The ethanol extract was suspended in 8L of distilled water, and extracted three times with equal volumes of petroleum ether, dichloromethane, ethyl acetate and n-butanol;
[0019] (3) The dichloromethane extract obtained in step (2) was separated by silica gel column chromatography, eluting with petroleum ether: ethyl acetate 8:1, 5:1, 3:1, 2:1,
[0020] (4) Petroleum ether:ethyl acetate 5:1 fraction obtained in the above step (3) was separated by semi-preparative HPLC chromatography, and eluted with acetonitrile / water gradient, acetonitrile / water 50:50~100:0, elution time 50 minutes, the flow rate is 8 mL / min, and the compound is obtained at a retention time of 25 minutes 1 , compound 2 was obtained with a retention time of 44 minutes.
[0021] The petrol...
Embodiment 2
[0031] (1) Dried the aboveground part (10kg) of Cymbidium chinensis was reflux extracted with 75% ethanol as the solvent, and the extract was recovered under reduced pressure to obtain the crude ethanol extract;
[0032] (2) Suspend the ethanol extract in 5L of distilled water, and extract twice with equal volumes of petroleum ether, dichloromethane, ethyl acetate and n-butanol;
[0033] (3) The dichloromethane extract obtained in step (2) was separated by silica gel column chromatography, and eluted with petroleum ether: acetone 8:1, 6:1, 4:1, 3:1,
[0034] (4) The petroleum ether: acetone 4:1 fraction obtained in the above step (3) was separated by semi-preparative HPLC chromatography, and eluted with methanol / water gradient, methanol / water 75:25~100:0, elution time 60 Minutes, the flow rate is 8 mL / min, and the compound is obtained at a retention time of 33 minutes 1 , Compound 2 was obtained with a retention time of 54 minutes.
[0035] The petroleum ether: acetone 9:1 f...
Embodiment 3
[0037] Example 3 Test of quinone reductase-inducing activity of compounds 1, 2 and 3
[0038] Experimental Materials:
[0039] (1) Main reagents and main equipment: calf serum (purchased from Hyclone); fetal bovine serum (TBD);
[0040] Tetramethylazolium salt (MTT) American Sigma (St. Louis, MO); Glucose-6-phosphate dehydrogenase (Shanghai Sangong); 96-well cell culture plate (Costar); cell line: mouse liver cancer cell Strain Hepa 1c1c7
[0041] experimental method:
[0042] (1) Cell culture:
[0043] Seed 10 per hole 4 Cells were grown for 24 hours in a culture medium containing 10% (v / v) fetal bovine serum, 0.01% penicillin G, 0.15% sodium bicarbonate, 0.01% streptomycin sulfate, the environmental conditions were 37 ℃, containing 5% CO 2 of humid air.
[0044] (2) Preparation of drugs
[0045] The compound to be tested was dissolved in DMSO to make a mother solution with a concentration of 50mmol / L, and stored at -20°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com