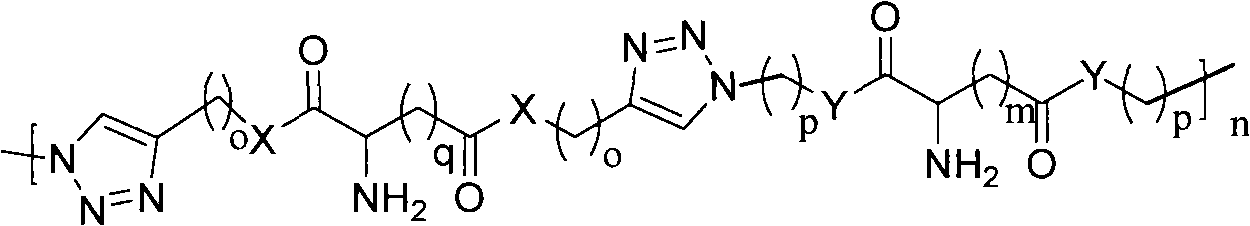
Cationic polymer and preparation method thereof
A technology of cationic polymers and compounds, applied in the field of cationic polymers and their preparation, can solve the problems of low molecular weight and complicated preparation process of ε-polylysine, and achieve the effect of simple preparation method and high molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
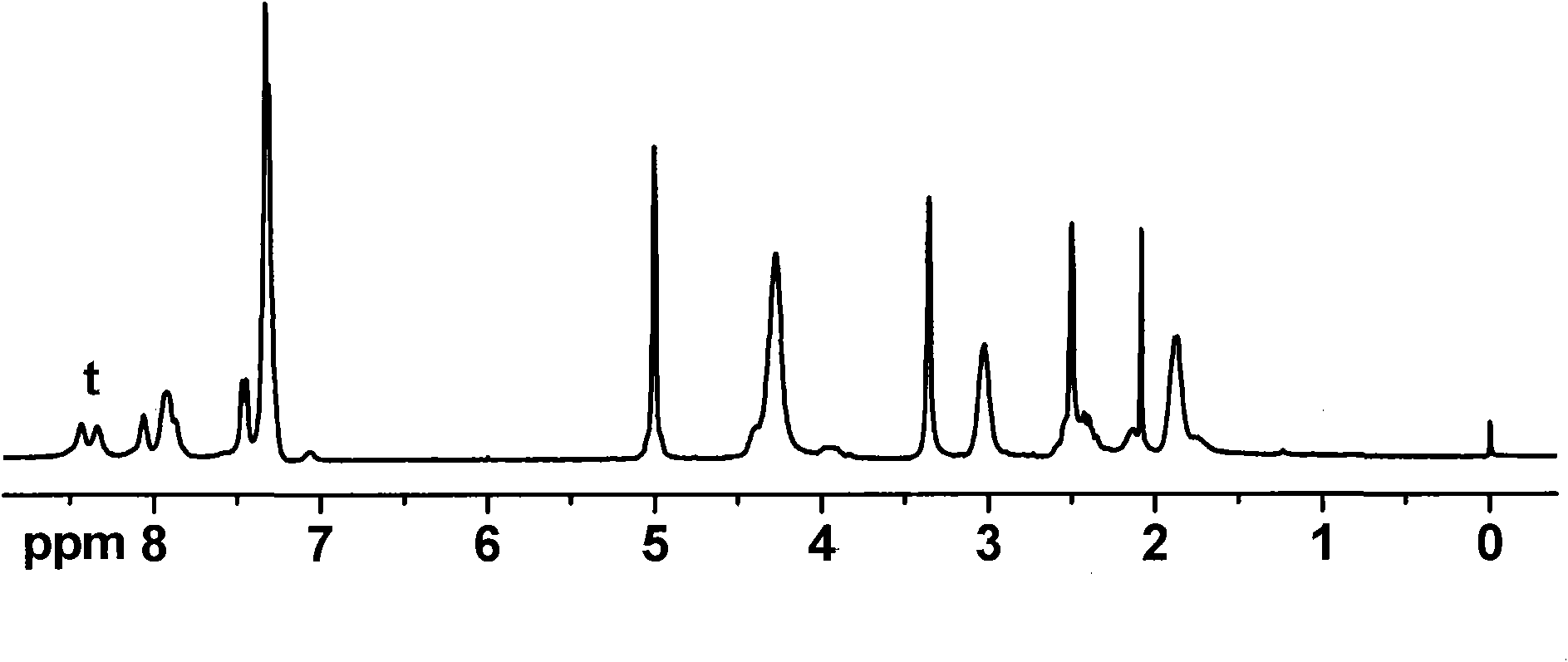
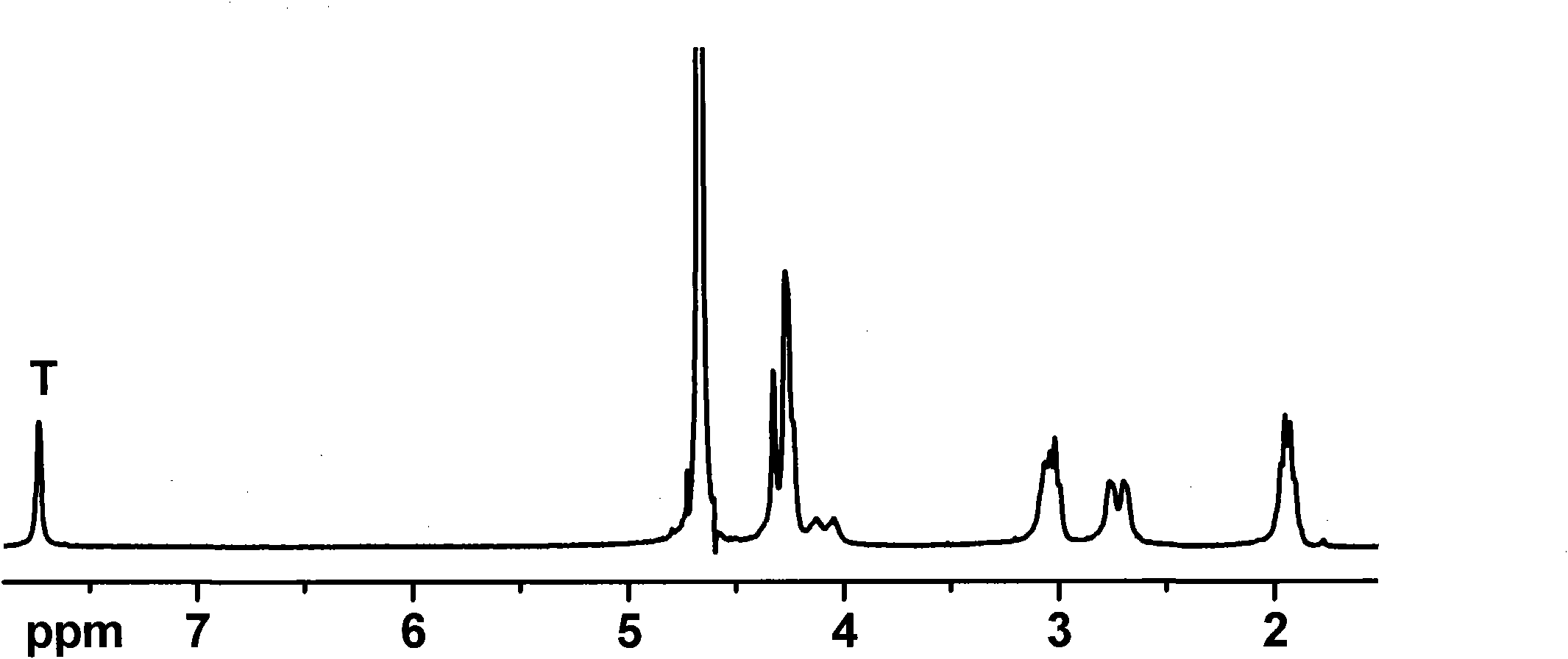
[0079] Example 1 Synthesis of aspartic acid dipropargylamide protected by benzyloxycarbonyl (Z-)
[0080] Disperse 2.66g of Z-protected aspartic acid (Z-Asp) into 60mL of dry tetrahydrofuran, add 8mL of dry triethylamine, stir and dissolve, add 2.8mL of propargylamine and 10.6g of Carter condensing agent (BOP reagent), The mixture was reacted at room temperature under nitrogen protection for 24 hours. After the reaction was completed, THF was concentrated, and the reaction mixture was dissolved in N, N-dimethylformamide (DMF), and dissolved in 1M KHSO 4 Settled in the aqueous solution, and the crude product was filtered with NaHCO 3 Washing with aqueous solution and water, settling and washing steps were repeated more than two times to completely remove impurities, and the product was vacuum-dried at 50° C. for 24 hours and then stored for later use. The yield was about 91%.
Embodiment 2
[0081] Example 2 Synthesis of aspartic acid diazide propionamide protected by benzyloxycarbonyl (Z-)
[0082]Disperse 1.33g of Z-protected aspartic acid (Z-Asp) into 30mL of dry tetrahydrofuran, add 4mL of dry triethylamine, stir to dissolve, add 1.5g of azidopropylamine and 5.3g of BOP reagent, and the mixture is React at room temperature for 24 hours under protection. After the reaction was completed, THF was concentrated, and the reaction mixture was dissolved in N, N-dimethylformamide (DMF), and dissolved in 1M KHSO 4 Settled in the aqueous solution, and the crude product was filtered with NaHCO 3 Washing with aqueous solution and water, settling and washing steps were repeated more than two times to completely remove impurities, and the product was vacuum-dried at 50° C. for 24 hours and then stored for later use. The yield was about 89%.
Embodiment 3
[0083] Embodiment 3 The synthesis of the exposed aspartic acid diazide propionamide of amino group
[0084] Disperse 1.17g of Boc-protected aspartic acid (Boc-Asp) into 30mL of dry dichloromethane, add 4mL of dry triethylamine, stir to dissolve, add 1.5g of azidopropylamine and 5.3g of BOP reagent, the mixture React at room temperature for 24 hours under the protection of nitrogen. After the reaction was completed, the solvent was drained, and the crude product was dissolved in 100 mL of ethyl acetate and washed with 1M KHSO 4 Aqueous solution, NaHCO 3 The aqueous solution, saline and water were extracted separately, and the organic phase was dried with magnesium sulfate and then spin-dried to obtain Boc-protected aspartic acid diazide propionamide with a yield of about 85%.
[0085] Dissolve 1.44 g of Boc-protected aspartic acid diazide propionamide in 30 mL of trifluoroacetic acid / chloroform (the volume ratio of trifluoroacetic acid and chloroform is 1:1), stir and react a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com