Method for preparing medicinal recombinant human interleukin-11
A technology for interleukin and fusion protein, which is applied in the field of preparation of pharmaceutical recombinant human interleukin-11, can solve the problems of uncontrollable degree of glycosylation, poor process stability, high process difficulty, etc., and achieves safety and effectiveness Sexual guarantee, quality controllable, good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
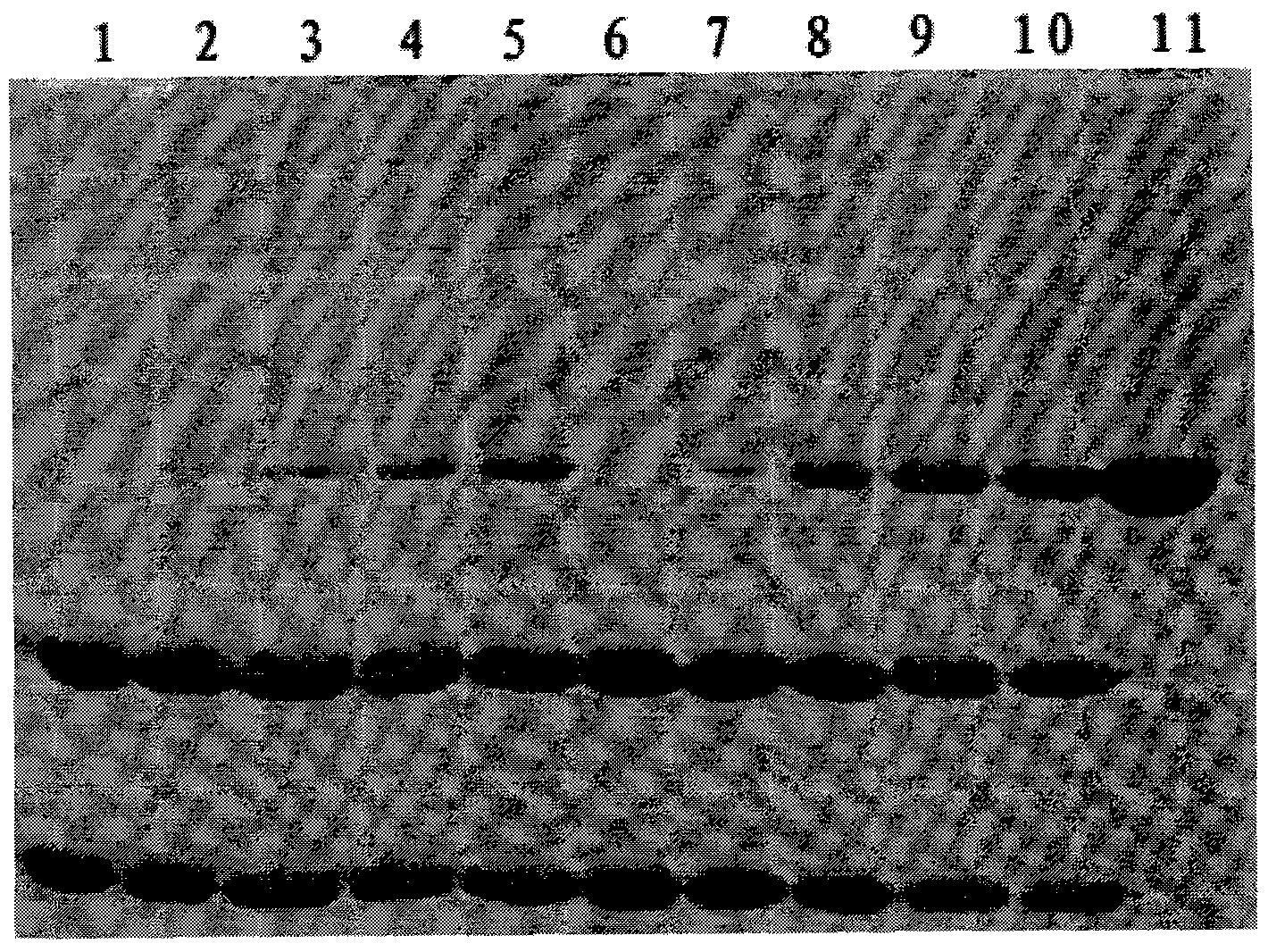
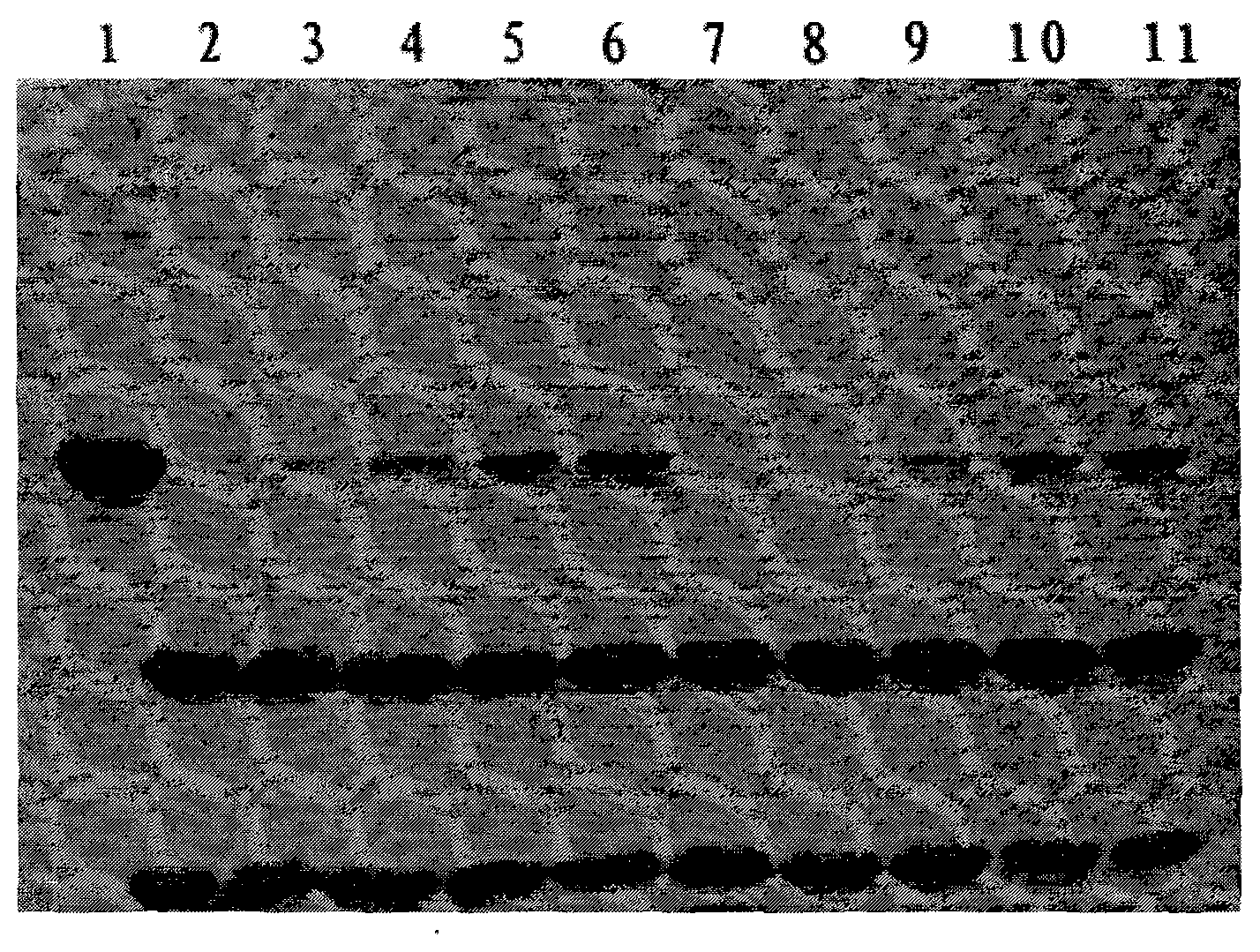
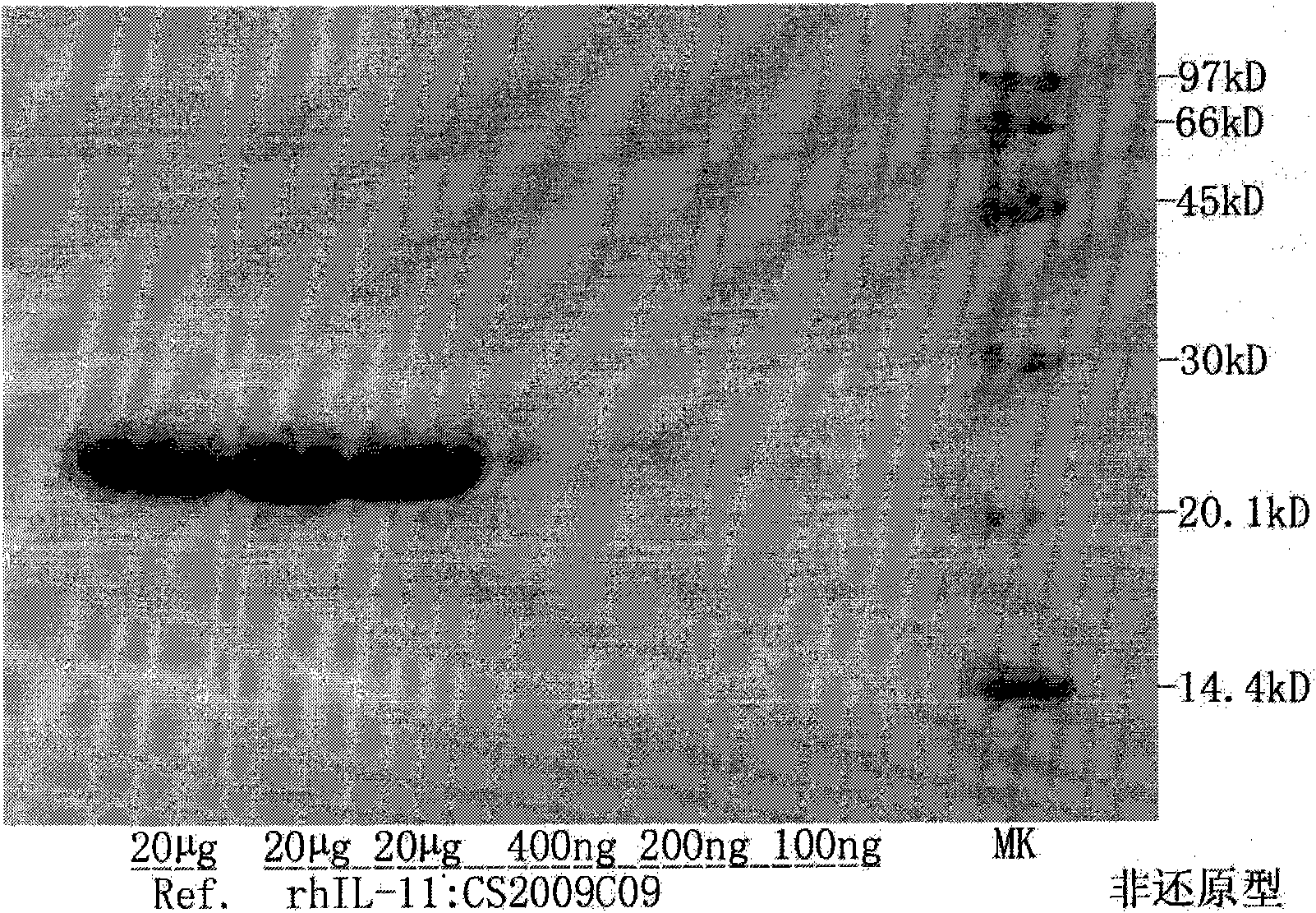
[0031] 1) Preliminary purification of rhIL-11
[0032] a. Bacterial cell disruption: The bacterial cells were resuspended with 50 mM Tris+500 mM NaCl, pH 8.0 buffer at a ratio of 1:10 (w / v). Ultrasonic crushing can be used for a small amount, and high-pressure crushing can be used for a large amount. The centrifuged supernatants of the cells suspended in the disrupted solution were compared using 100-900 Psi pressure. The result shows: 200Psi has higher yield and higher purity.
[0033] b. Separation by chelating column: According to the characteristics of MB residues in the fusion protein, Chelating Sepharose FF was selected as the filler for preliminary purification. Use 100mM imidazole to wash away impurity proteins, and the elution condition of the target protein is 50mM Tris-Cl, 285-325mM imidazole, 1% Tween 80 gradient elution, the purification results show that this step of purification can make the target fusion protein The purity is as high as more than 90%, which ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com