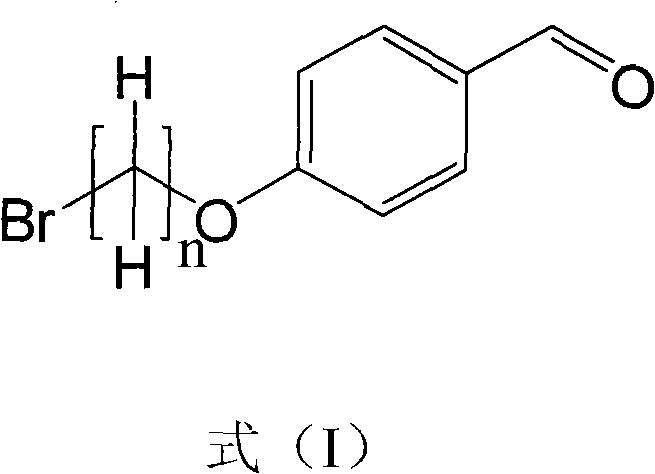
Method for synthesizing mitochondria targeted spinning scavenger MitoPBNs (spinning probe)
A synthesis method and compound technology are applied in the field of synthesizing mitochondria-targeted spin trapping agent MitoPBN (spin probe) series compounds, which can solve the problems of low product purity, cumbersome preparation procedures, harsh reaction conditions and the like, and achieve cheap raw materials. The effect of easy availability, high product purity, and short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment M
[0022] Synthesis of Example MitoPBN
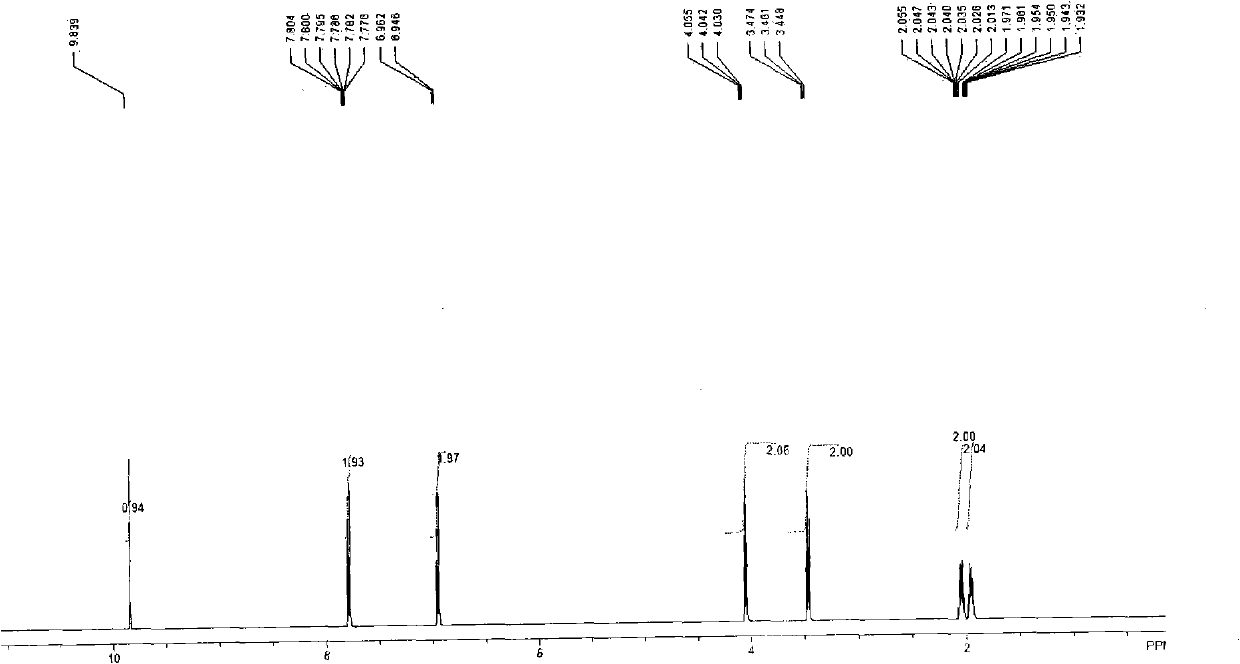
[0023] 1) Synthesis of 4-(4-bromobutoxy)benzaldehyde: Add 2.0 grams (16.4mmol) of p-hydroxybenzaldehyde in a 250mL reaction flask, 4.5 grams (32.8mmol, 2eq) of anhydrous potassium carbonate, and 2.5mL of 1,4-Dibromobutane and 50 ml of DMF were reacted for 12 hours, the reaction solution was poured into 150 ml of ice water, extracted with ethyl acetate (50 ml x 4), the organic phases were combined and washed once with 100 ml of water. The organic phase was dried over anhydrous magnesium sulfate, filtered, and distilled under reduced pressure to obtain a concentrated crude product. The crude product was separated and purified by silica gel column chromatography (eluent: ethyl acetate / petroleum ether=1 / 3). 3.1 g of the product was obtained with a yield of 73%. 1 H NMR (400MHz, CDCl 3 ): δ9.84(s, 1H), 7.78(d, J=5.6Hz, 2H), 6.95(d, J=3.2Hz, 2H), 4.04(t, 2H), 3.46(t, 2H), 2.01 -2.05 (m, 2H), 1.93-1.97 (m, 2H).
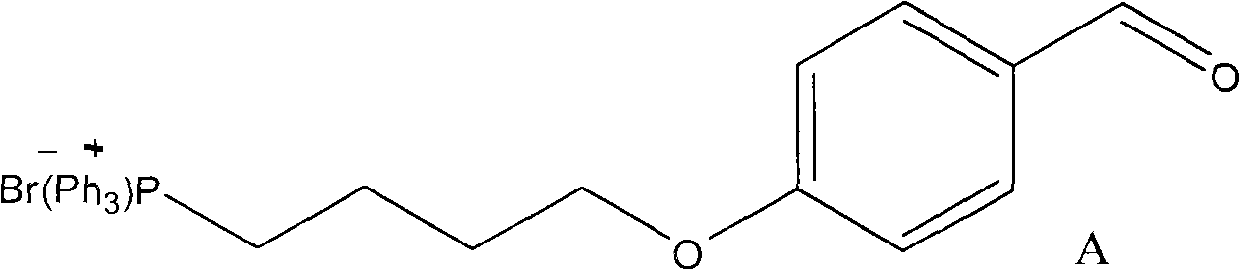
[0024] 2) Synthesis of quate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com