Human complement fragment C5a gene as well as clone and recombinant protein expression method thereof
A protein, expression vector technology, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Construction and protein expression of C5a gene
[0021] 1. Amplification of C5a gene
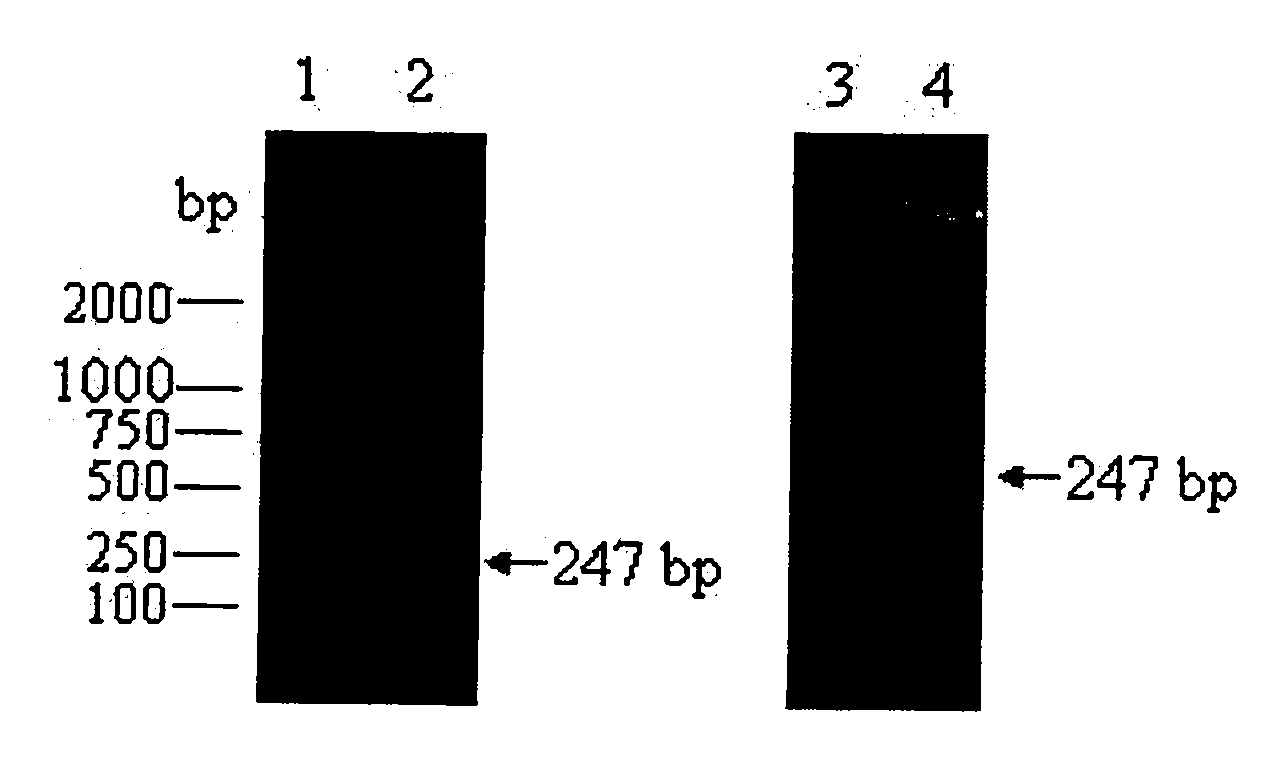
[0022] According to the mRNA sequence of human C5a (DQ400449) provided in Genebank, and according to the preferred codons of Escherichia coli, the designed and synthesized figure 1 The 6 primer fragments shown are named P1 to P6 respectively, and the primers were synthesized by Dalian Bao Biological Company.
[0023] BamH I and Xho I restriction sites were introduced into the 5' ends of the P5 and P6 primers, respectively, and the human C5a gene was constructed by overlapping PCR method. The first round of PCR paired P1 and P2 to generate product P12, the second round of PCR used P12 as template, P3 and P4 as primers to generate product P34, and the third round of PCR used P34 as template, P5 and P6 as primers to generate C5a gene fragment . The PCR reaction was carried out according to the conventional method, and the results were observed by agarose gel electrophoresi...
Embodiment 2
[0034] Example 2: Purification and identification of C5a protein
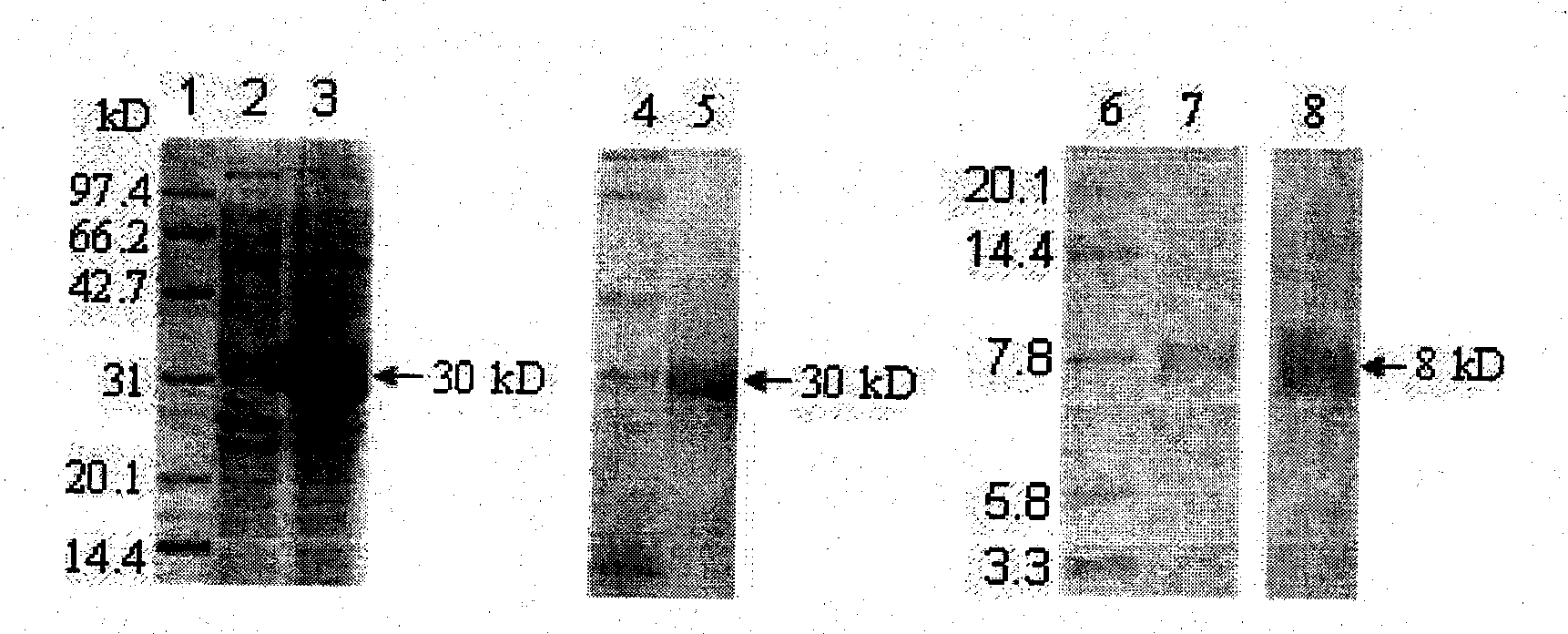
[0035] 1. Purification and quantification of Trx-C5a fusion protein
[0036] The induced bacteria were collected, washed once with PBS, the wet bacterial pellet was resuspended with PBS, placed in an ice bath, sonicated, centrifuged at 12,000 rpm for 20 min, and the supernatant was the protein sample to be purified. Purify with Ni Sepharose 6Fast Flow purification column (purchased from GE company), connect the purification column to Akta purifier, use Binding Buffer (0.02M PB, 0.5M NaCl, 30mM imidazole, pH7.4) to balance 5 column volumes, The protein sample to be purified was loaded at a speed of 1 ml / min, washed with Binding Buffer to the baseline after loading, and eluted with protein elution buffer (0.02M PB, 0.5M NaCl, 200mM imidazole, pH7.4), and the protein was collected Peak, the eluate was dialyzed in PBS at 4°C overnight, and a small sample was taken for 12% SDS-PAGE electrophoresis to analyze the pu...
Embodiment 3
[0044] Example 3: Biological activity of C5a protein after purification
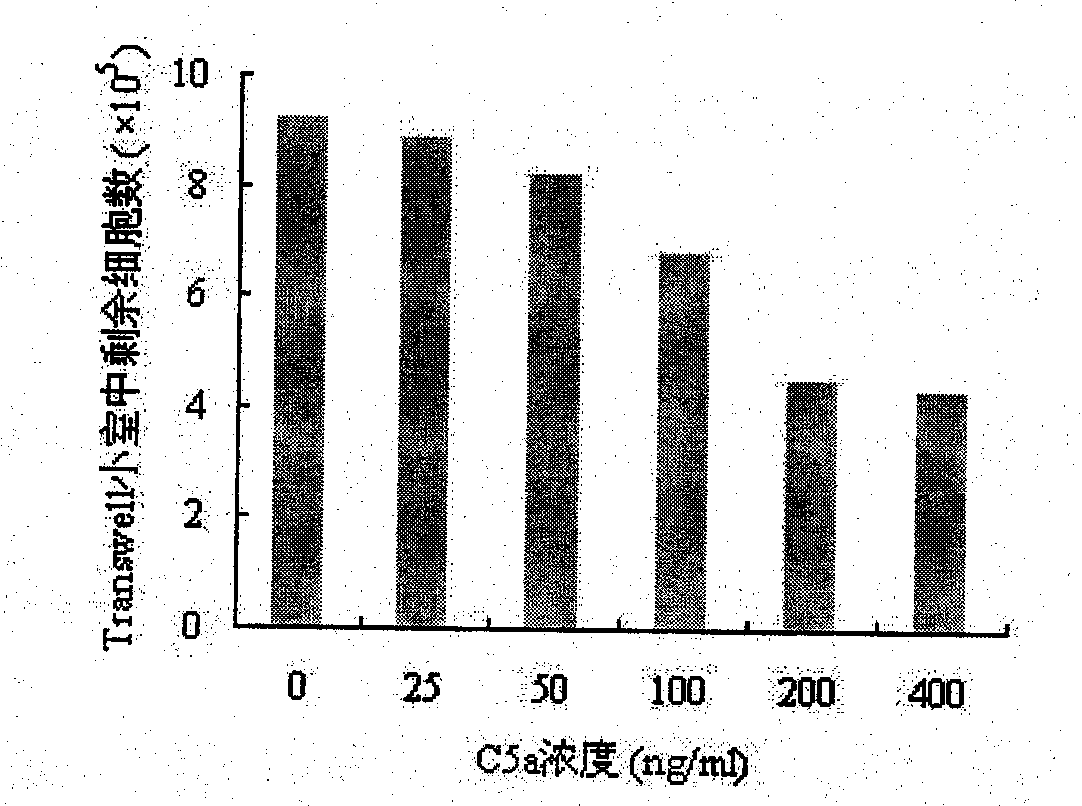
[0045] 1. Chemotaxis of C5a protein to human neutrophils
[0046] Isolation of human peripheral blood neutrophils. Take 10ml of heparin for anticoagulation, add 2.5ml of 6% dextran physiological saline, mix well, and let stand at room temperature for 30-45min to make red blood cells sink. Take the supernatant, slowly add an equal volume of polysucrose-diatrizoate to the surface of the separation liquid, and centrifuge at 500 rpm for 30 min. Precipitate with 0.83% NH 4 The erythrocytes were destroyed by the Cl solution. After centrifugation, the pellet was washed twice with PBS. The pellet was resuspended in serum-free 1640 and adjusted to a concentration of 5×10 6 / ml spare.
[0047] Add 350 μl of serum-free 1640 to the wells of a 24-well cell culture plate, and add C5a protein at final concentrations of 0 ng / ml, 25 ng / ml, 50 ng / ml, 100 ng / ml, 200 ng / ml and 400 ng / ml, respectively, The Transwell cha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com