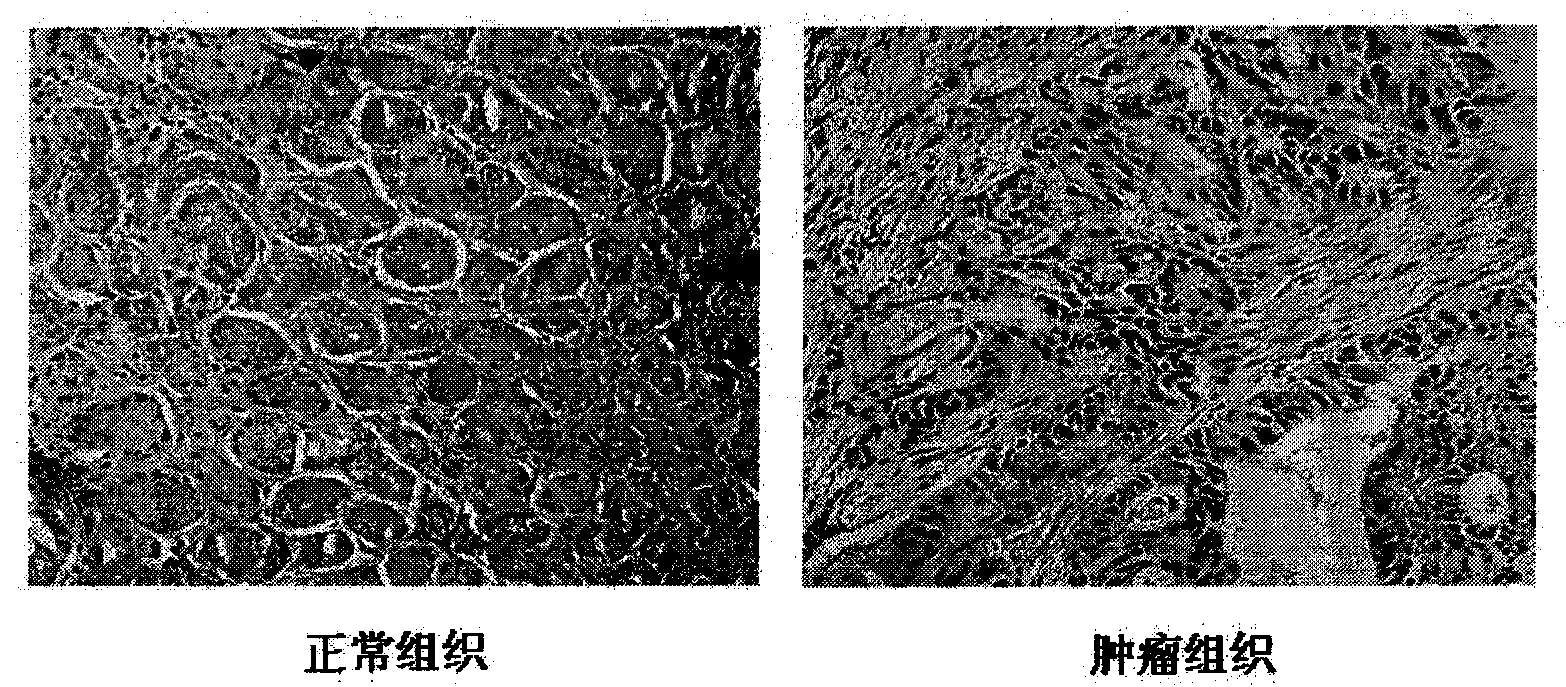
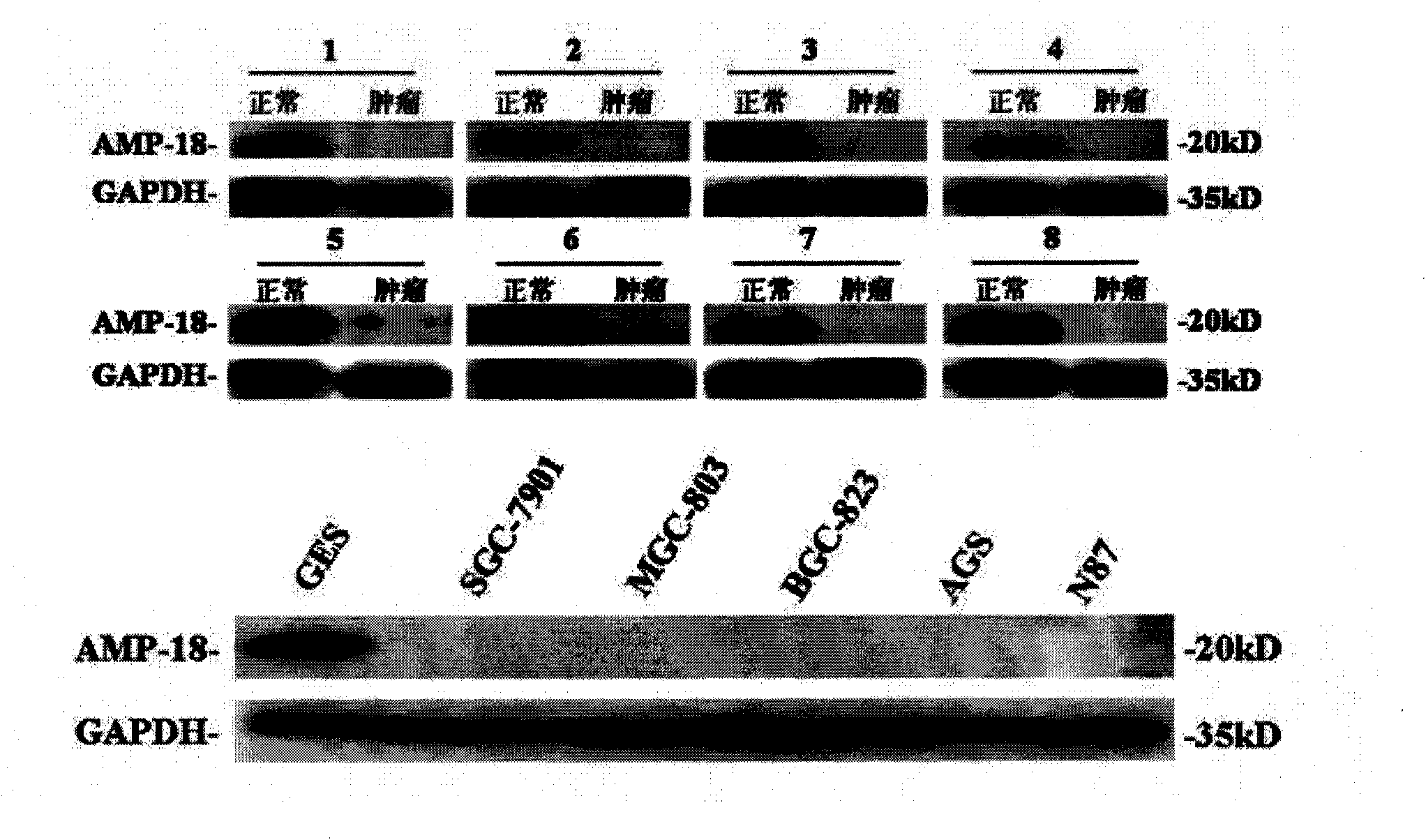
Hybrid tumor generating anti-AMP-18 (Antrum Mucosalprotein-18) monoclonal antibody as well as anti-AMP-18 monoclonal antibody and application thereof in gastric carcinoma detection
An AMP-18, monoclonal antibody technology, applied in anti-animal/human immunoglobulins, microorganisms, measuring devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Preparation of AMP-18 antigen
[0016] AMP-18 antigen is based on normal gastric tissue cDNA as a template, and specific primers are designed according to the AMP-18 sequence (SEQID No.1), and both ends of the primers are connected to BamHI and SalI restriction sites
[0017] Upstream primer: 5′-TGAAGGATCCAACTATAATATCAACGTC-3′ (SEQ ID No.2)
[0018] Downstream primer: 5'-AAATGTCGACGTTTCTCCACCGTGTCTCC-3' (SEQ ID No.3)
[0019] The gene that removed the AMP-18 signal peptide sequence was amplified by PCR (PCR parameters: 95°C for 5 minutes; 95°C for 30s, 55°C for 30s, 72°C for 60s, a total of 40 cycles; 72°C and then extended for 10 minutes) by BamHI and SalI double enzymes After cutting, it was ligated with pQE30a, and the competent cell JM109 was chemically transformed, and the single clone was picked to extract the plasmid for sequencing, and the sequence was verified to be correct. Select positive bacteria with correct sequencing and induce expression wit...
Embodiment 2
[0021] Embodiment 2: Preparation of anti-AMP-18 hybridoma
[0022] 1) immunity
[0023] The AMP-18 protein purified by Ni-NAT affinity chromatography was subcutaneously immunized in the abdomen (20 μL serum was taken from the orbit before immunization as a negative control) 4-6 week old female Balb / c mice; the dose was 60 μg per mouse Protein+normal saline to 200μL+CFA200μL. Subcutaneous booster immunization once every 14 days, the dose was 30 μg protein + normal saline to 200 μL + IFA 200 μL per mouse. 7 days after the third booster immunization, blood was taken from the orbit to measure the titer. For those who met the requirements, 50 μg protein + normal saline was added to 100 μL; the tail vein was injected, and it was fused after 3 days.
[0024] 2) Fusion
[0025] The freshly excised mouse spleen was crushed and filtered on a cell sieve, mixed with sp2 / 0 cells at a ratio of 1:5, and centrifuged at 1500 rpm for 5 minutes. Put the centrifuge tube containing the centrif...
Embodiment 3
[0034] Example 3: Preparation of anti-AMP-18 monoclonal antibody by hybridoma cell line with deposit number CGMCC 2839
[0035] The inventor used the hybridoma (70110-4, date of deposit on December 30, 2008, deposit number CGMCC 2839, place of deposit: CGMCC, General Microbiology Center, China Committee for Culture Collection of Microorganisms) to prepare mouse anti-AMP-18 monoclonal Antibody, the specific operation steps are as follows:
[0037] Quickly take out the cryopreservation tube from the -80°C refrigerator or liquid nitrogen tank; quickly put it into a 37°C water bath and stir quickly to make the cryopreservation solution melt into a liquid within 2 minutes. Add 3mL of serum medium to a 15mL centrifuge tube, draw the cryopreserved solution into the centrifuge tube, and centrifuge at 1500 rpm for 5 minutes. Discard the supernatant, suspend the cells with complete medium, and culture in a 6-well plate (3 mL) or bottle (5 mL).
[0038] 2) As...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com