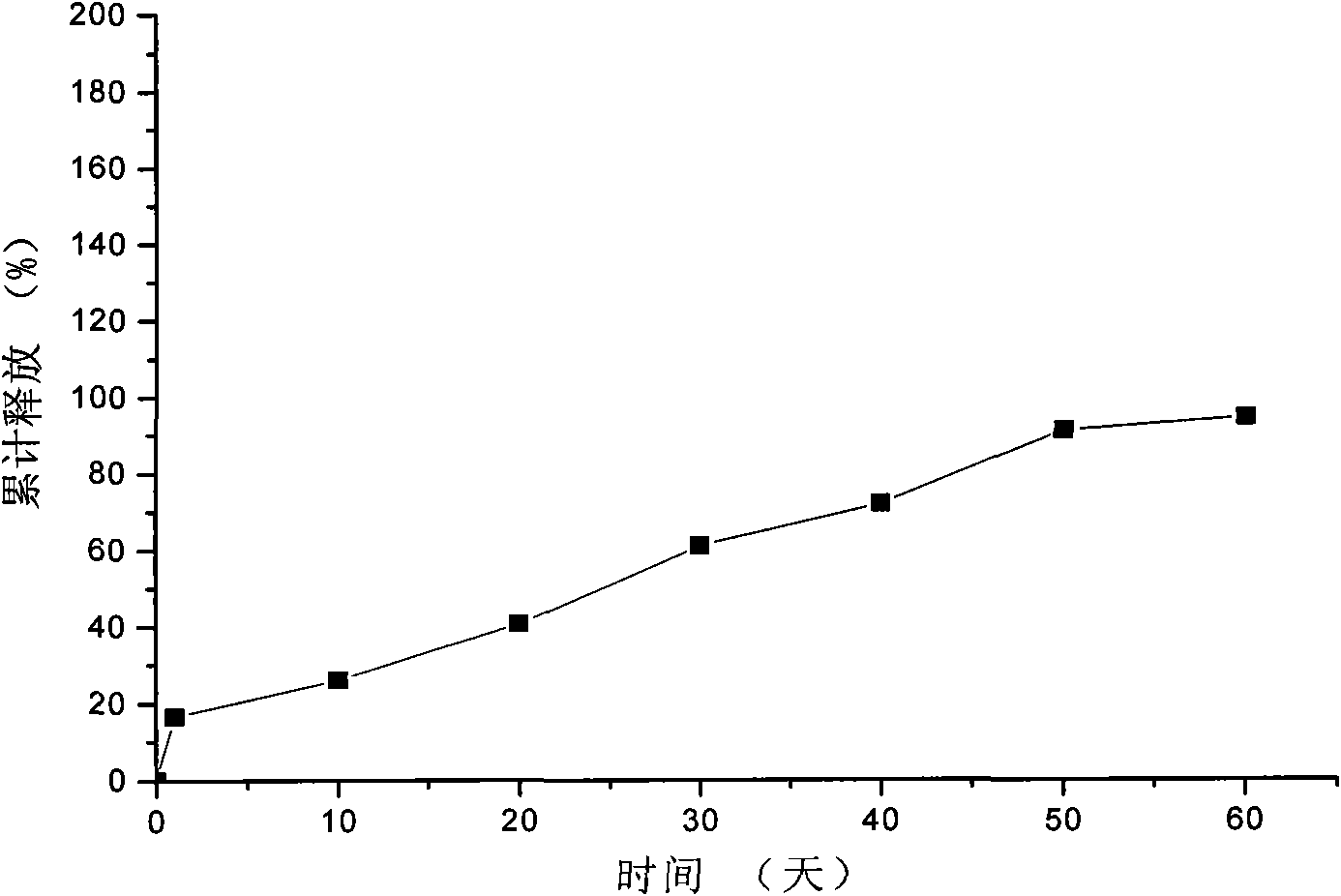Lanreotide long-acting injection microsphere preparation and preparation method thereof
A technology of lanreotide and microspheres, which is applied in the field of long-acting lanreotide injection microsphere preparations and its preparation, can solve the problems of complex process of long-acting sustained-release preparations, difficulties in comprehensive clinical promotion, poor compliance of treatment, etc., and achieve Improve medication compliance, reduce treatment burden, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Dissolve gelatin with water to make a 7.5% gelatin solution, add 80g of lanreotide hydrochloride to 200mL of the above gelatin solution, heat on a water bath at 40°C to completely dissolve the drug, and use it as the inner water phase for later use;
[0039] 2. Dissolve 800g polylactic acid-glycolic acid copolymer (the molar ratio of lactide to glycolide monomer is 85 / 15) in 1000mL acetone, as the organic phase, and set aside;
[0040] 3. Add the organic phase of step 2 to the inner water phase of step 1, mix the inner water phase and the organic phase, and ultrasonically emulsify at 45°C to obtain colostrum;
[0041] 4. Cool the above-mentioned colostrum to 12°C, add it to 4000mL of 4% polyvinyl alcohol that has been cooled to 12°C, and stir at a high speed of 7500r / min for 2min to obtain double emulsion;
[0042] 5. Add the double emulsion to 2000mL 0.1% polyvinyl alcohol solution, heat in a water bath at 30°C, stir at a low speed of 75r / min for 3h, then evaporate ...
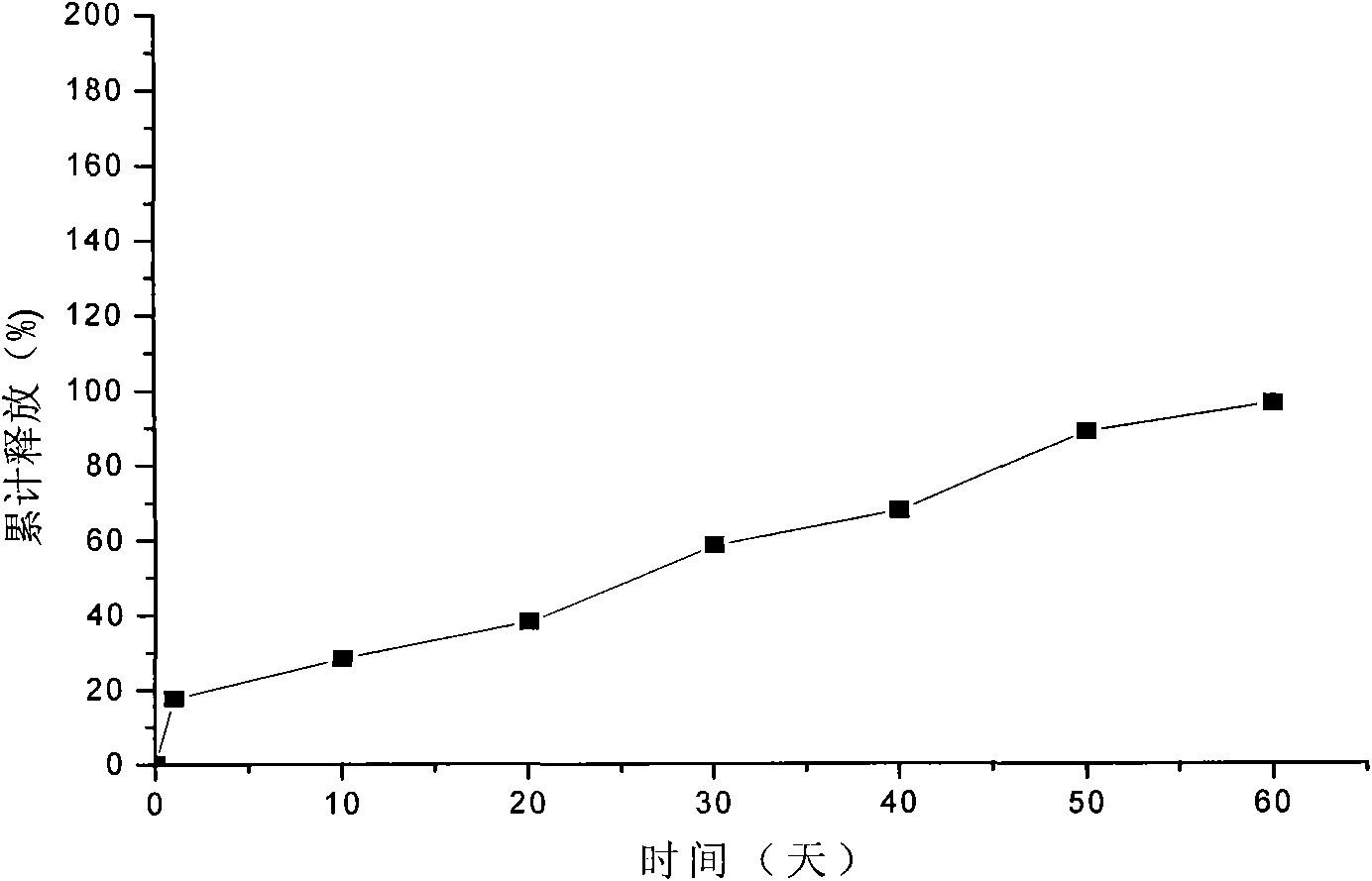
Embodiment 2
[0046] 1. Dissolve gelatin with water to make a 7.5% gelatin solution, add 100g of lanreotide acetate to 250mL of the above gelatin solution, heat on a water bath at 55°C to completely dissolve the drug, and use it as the inner water phase for later use;
[0047] 2. Dissolve 1000g of polylactic acid-glycolic acid copolymer (the molar ratio of lactide to glycolide monomer is 85 / 15) in 1500mL of acetone, as the organic phase, and set aside;
[0048] 3. Add the organic phase of step 2 to the inner water phase of step 1, mix the inner water phase and the organic phase, and ultrasonically emulsify at 40°C to obtain colostrum;
[0049] 4. Cool the above-mentioned colostrum to 16°C, add it to 4000mL of 4% polyvinyl alcohol that has been cooled to 16°C, and stir at a high speed of 5000r / min for 2min to obtain double emulsion;
[0050] 5. Add the double emulsion to 2000mL 0.1% polyvinyl alcohol solution, heat in a water bath at 30°C, stir at a low speed of 50r / min for 3h, then evaporat...
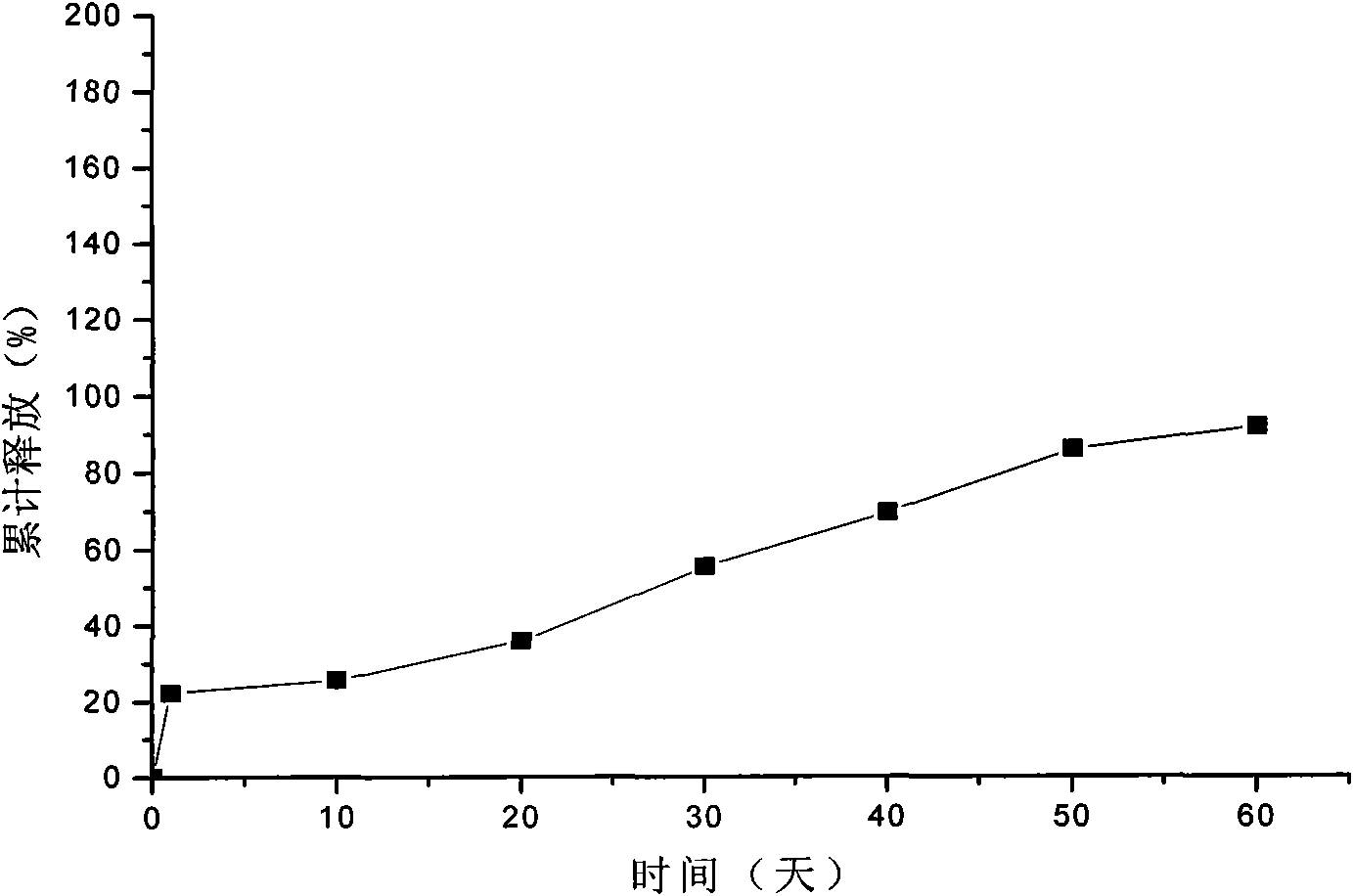
Embodiment 3
[0054] 1. Dissolve hydroxypropyl cellulose with water to make a 5% hydroxypropyl cellulose solution, add 150g of lanreotide acetate to 400mL of the above hydroxypropyl cellulose solution, and heat on a water bath at 70°C to make the drug completely Dissolved, as the inner water phase, for subsequent use;
[0055] 2. Dissolve 1500g of polylactic acid-glycolic acid copolymer (the molar ratio of lactide to glycolide monomer is 85 / 15) in 2000mL of acetone, as the organic phase, and set aside;
[0056] 3. Add the organic phase of step 2 to the inner water phase of step 1, mix the inner water phase and the organic phase, and ultrasonically emulsify at 35°C to obtain colostrum;
[0057] 4. Cool the above-mentioned colostrum to 14°C, add it to 4000mL of 4% polyvinyl alcohol that has been cooled to 14°C, and stir at a high speed of 6500r / min for 2min to obtain double emulsion;
[0058] 5. Add the double emulsion to 2000mL 0.1% polyvinyl alcohol solution, heat in a water bath at 30°C, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com