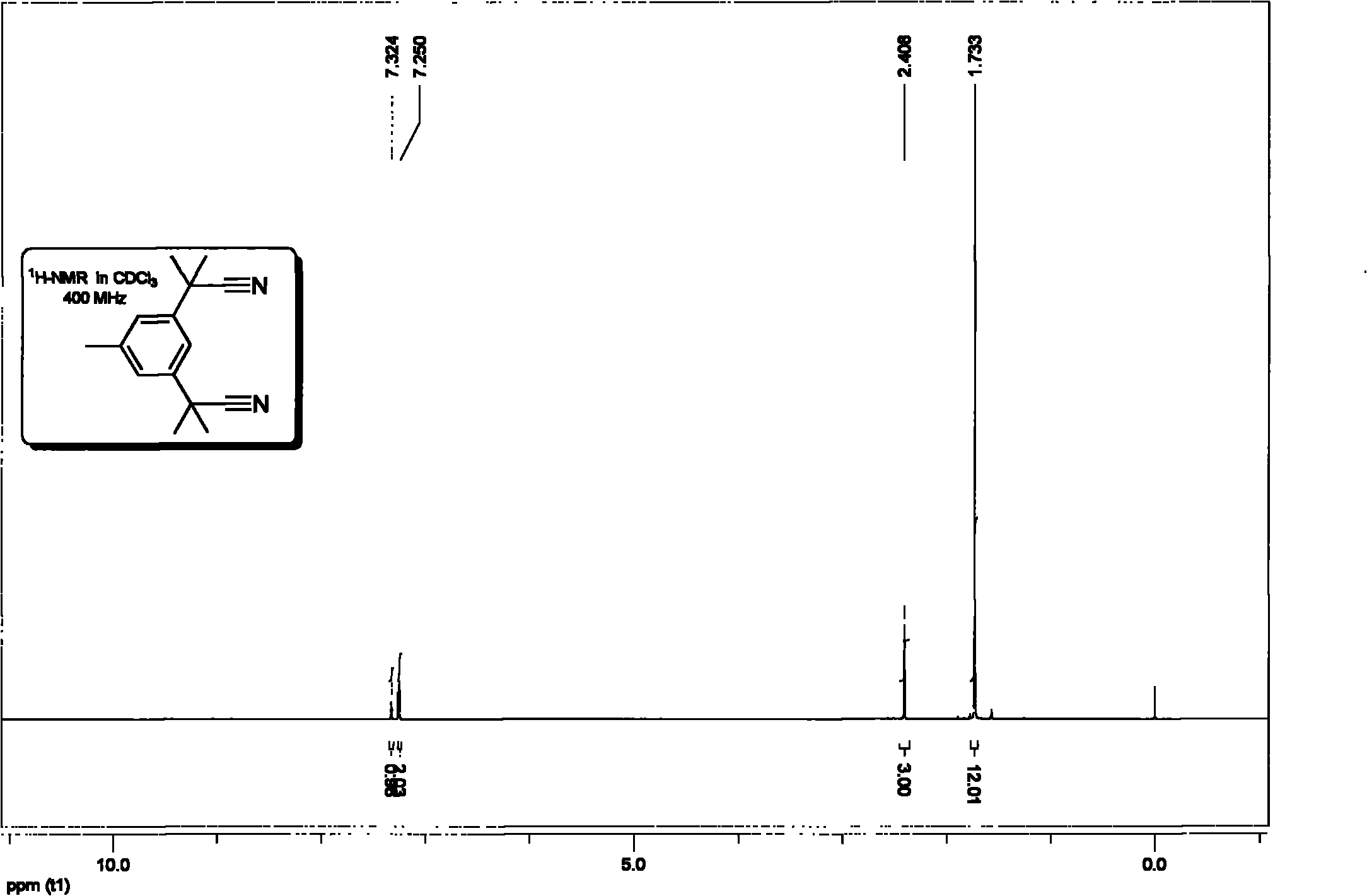
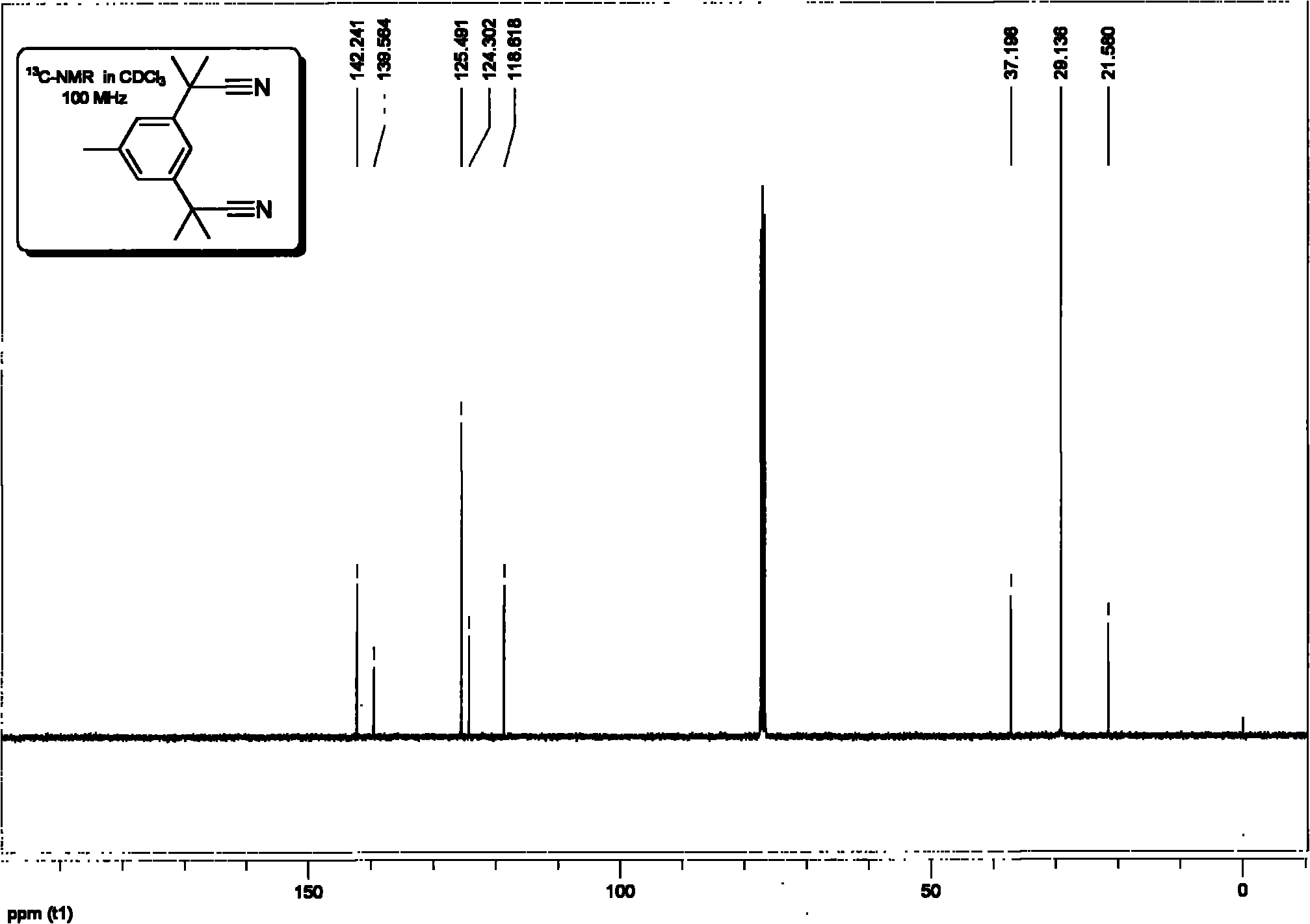
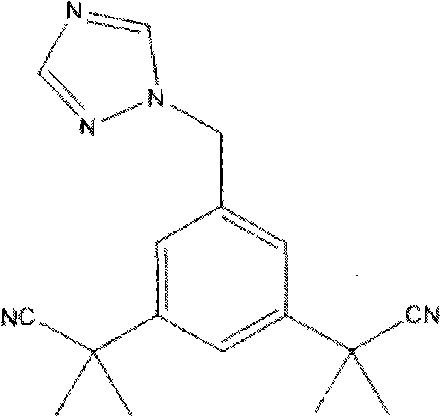
Method for synthesizing anastrozole intermediate-2,2'-(5-methyl-1,3-phenylene)-bis-(2-methyl propionitrile)
A technology of anastrozole and methylpropionitrile, applied in the field of compound synthesis, can solve the problems of difficult synthesis of starting materials and low yield of intermediates, and achieve the effect of easy synthesis, high yield, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) Preparation of ethyl 2-cyano-2-methylpropionate
[0057] Add ethyl cyanoacetate (4.52g, 40mmol), DBU (22.07g, 88mmol) and 50mL DMF to a 150ml round bottom flask equipped with a magnet. After stirring in an ice-water bath for 10 minutes, iodomethane (12.49 g, 88 mmol) was slowly added dropwise. After the dropwise addition, the round-bottomed flask was covered with a lid, the ice-water bath was removed, and the reaction was stirred at room temperature for 2 h. After the reaction, the solution in the round bottom flask was transferred to a 250mL separatory funnel, 80mL of ethyl acetate was added, the organic phase was extracted twice with 80mL of water, and once with 80mL of saturated saline. After the extraction, the organic phase was dried with anhydrous magnesium sulfate for 20 minutes, filtered, and the solvent ethyl acetate was removed by a vacuum rotary evaporator, and then separated by a simple chromatographic column to obtain a light yellow liquid with a yield...
Embodiment 2
[0064] (1) Preparation of sodium cyanoacetate
[0065] method 1:
[0066] Sodium hydroxide (1.60 g, 40 mmol) and 50 mL of ethanol were added to a 150 mL round bottom flask equipped with a magnet, and cyanoacetic acid (3.40, 40 mmol) and 30 mL of ethanol were added to another 100 mL round bottom flask. Add the ethanol solution of cyanoacetic acid dropwise to the ethanol solution of sodium hydroxide under stirring, and continue stirring for 20 minutes after the dropwise addition is completed. After the reaction was completed, ethanol and a small amount of water produced were removed by a vacuum rotary evaporator, filtered, and washed three times with a small amount of ether to obtain a white solid with a yield of 98%.
[0067] Method 2:
[0068] Sodium hydroxide (1.60 g, 40 mmol) and 50 mL of ethanol were added to a 150 mL round bottom flask equipped with a magnet, and ethyl cyanoacetate (4.52 g, 40 mmol) and 30 mL of ethanol were added to another 100 mL round bottom flask. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com