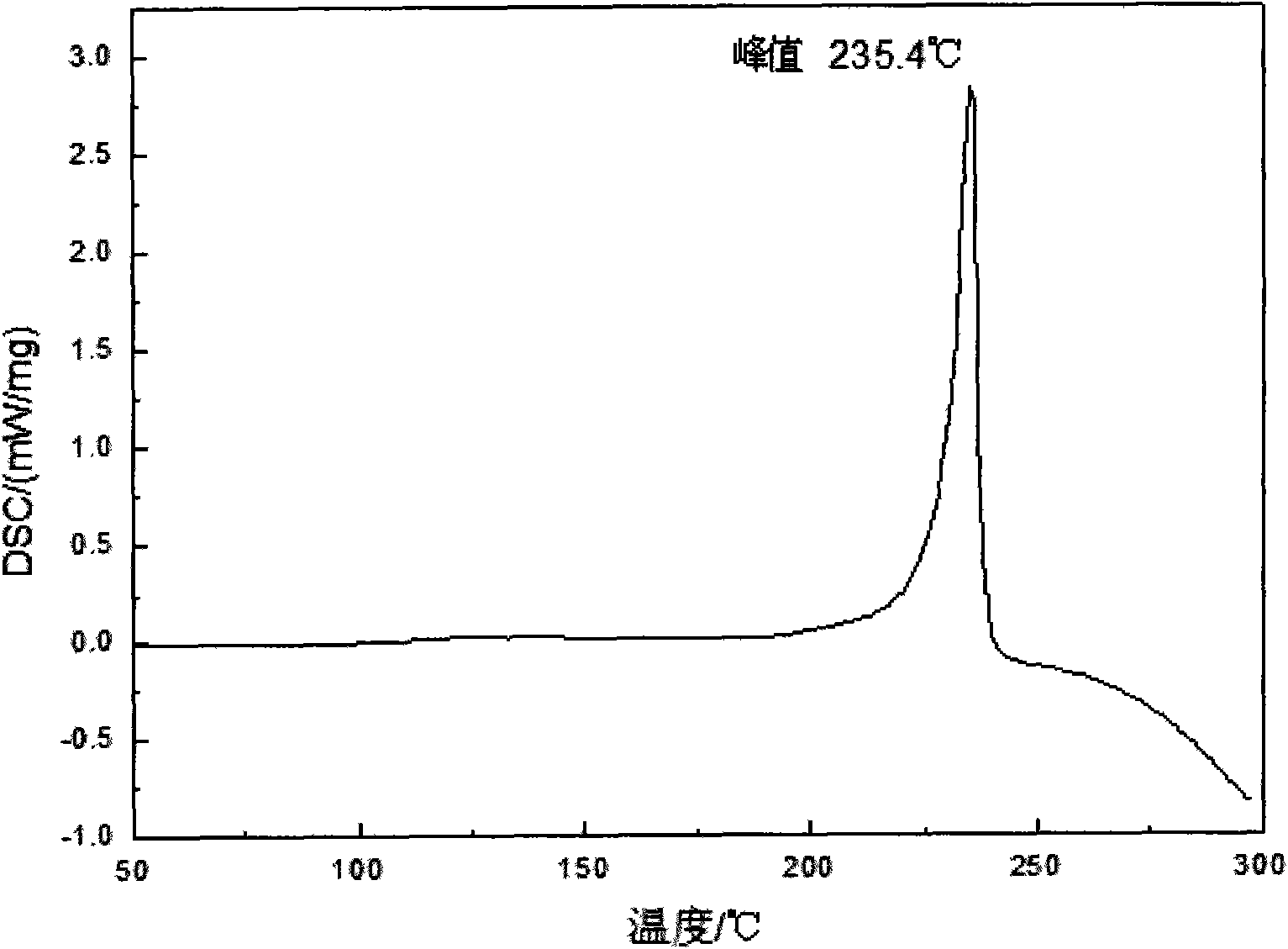
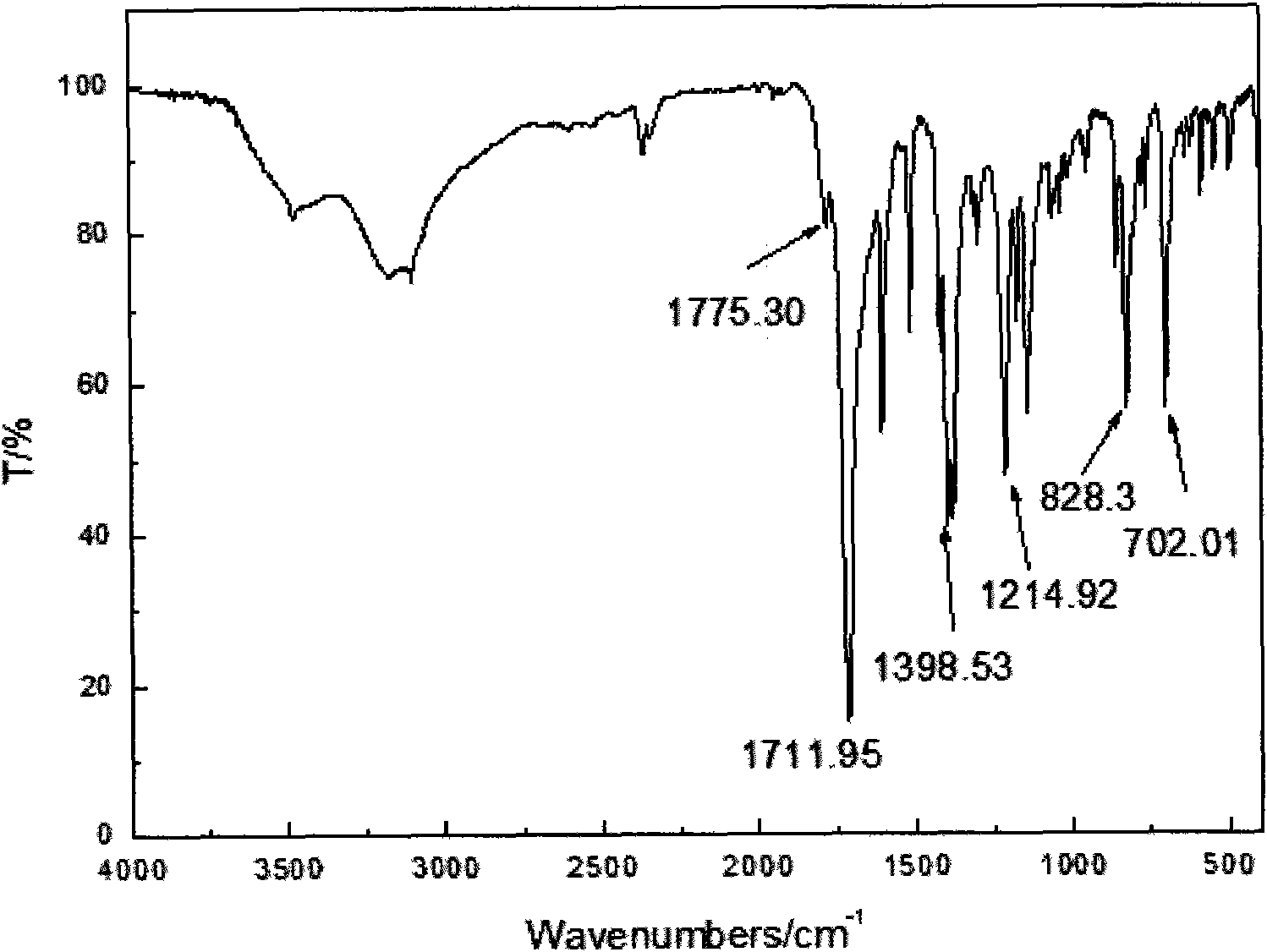
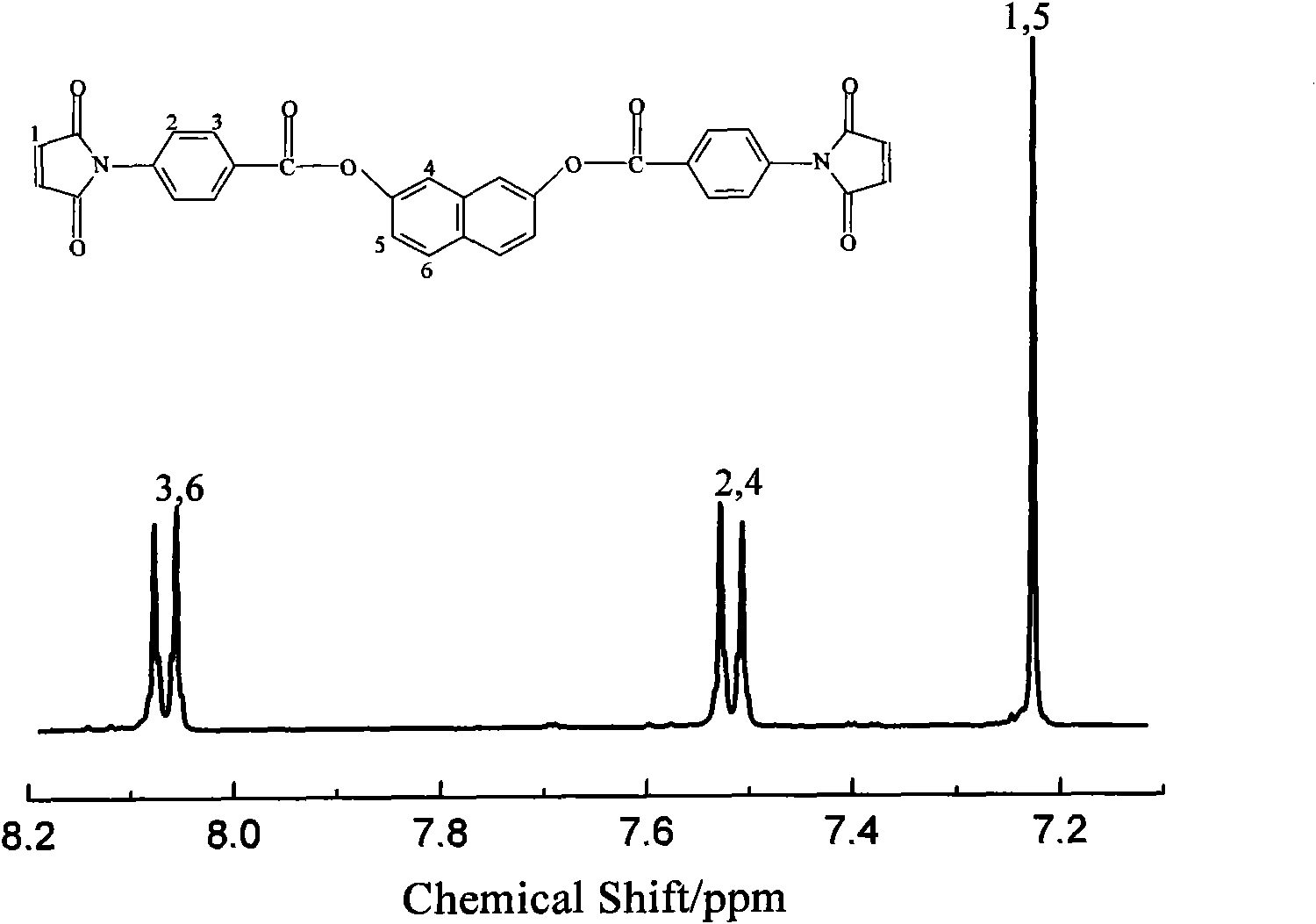
Naphthalene ring and ester bond structure-containing bismaleimide type compound and preparation method thereof
A technology of bismaleimide and maleimide benzoic acid, which is applied in the field of organic compounds and its preparation, can solve the problems of material rigidity and heat resistance decline, slow down the thermal decomposition speed, improve toughness, Unified effect of heat resistance and toughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]Add 10g of maleic anhydride, 14g of 4-aminobenzoic acid and 500ml of glacial acetic acid into a 1000ml four-necked flask, stir and heat, and react under reflux for 5-6 hours. Stop heating, cool the reaction system, filter to obtain a light yellow solid, wash it with water, and dry it under vacuum at 80°C to obtain a light yellow powder product; Crystallization affords 4-maleimide benzoic acid. According to the charging capacity of maleic anhydride, the theoretical output and the actual output of 4-maleimide benzoic acid, the calculated yield of 4-maleimide benzoic acid is 88.9%, and the product is light yellow powder.
[0042] In pass have N 2 Add 600ml of benzene and 50g of 4-maleimide benzoic acid into a 1000ml four-neck flask, stir to dissolve, add 4 to 5 drops of pyridine as a catalyst, and add 100ml of SOCl dropwise at 5°C (ice bath) 2 After the dropwise addition, react at room temperature for 1 to 2 hours, raise the temperature to reflux state and continue to rea...
Embodiment 2
[0045] Add 10g of maleic anhydride, 14g of 4-aminobenzoic acid and 500ml of glacial acetic acid into a 1000ml four-necked flask, stir and heat, and react under reflux for 5-6 hours. Heating was stopped, the reaction system was cooled, and a light yellow solid was obtained by filtration, washed with water, and dried in vacuum at 80° C. to obtain a light yellow powder product. Recrystallize with ethanol-water (volume ratio 3:1) mixed solvent to obtain 4-maleimide benzoic acid. According to the charging capacity of maleic anhydride, the theoretical output and the actual output of 4-maleimide benzoic acid, the calculated yield of 4-maleimide benzoic acid is 88.9%, and the product is light yellow powder.
[0046] 50g of 4-maleimide benzoic acid, 100ml SOCl 2 Add 500ml of acetonitrile into a four-neck flask and stir, add 2ml of dimethylformamide as a catalyst, react at 40°C for 30min, then heat to reflux for 2-4 hours until the solution becomes uniform and transparent, distill The...
Embodiment 3
[0049] Add 10g of maleic anhydride, 14g of 4-aminobenzoic acid and 500ml of glacial acetic acid into a 1000ml four-necked flask, stir and heat, and react under reflux for 5-6 hours. Heating was stopped, the reaction system was cooled, and a light yellow solid was obtained by filtration, washed with water, and dried in vacuum at 80° C. to obtain a light yellow powder product. Recrystallize with ethanol-water (volume ratio 3:1) mixed solvent to obtain 4-maleimide benzoic acid. According to the charging capacity of maleic anhydride, the theoretical output and the actual output of 4-maleimide benzoic acid, the calculated yield of 4-maleimide benzoic acid is 88.9%, and the product is light yellow powder.
[0050] In pass have N 2 Add 600ml of benzene and 50g of 4-maleimide benzoic acid into a 1000ml four-neck flask, stir to dissolve, a few drops of pyridine as a catalyst, keep at 5°C (ice bath) and dropwise add 100ml of SOCl 2 After the dropwise addition, react at room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com