Application of Xyloketal B in preparing antiatherosclerotic medicaments
A technology for atherosclerosis and atherosclerosis, which is applied in drug combinations, anti-toxins, and pharmaceutical formulas to achieve good medicinal prospects, strong pharmacological effects, and the effect of reducing macrophage deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
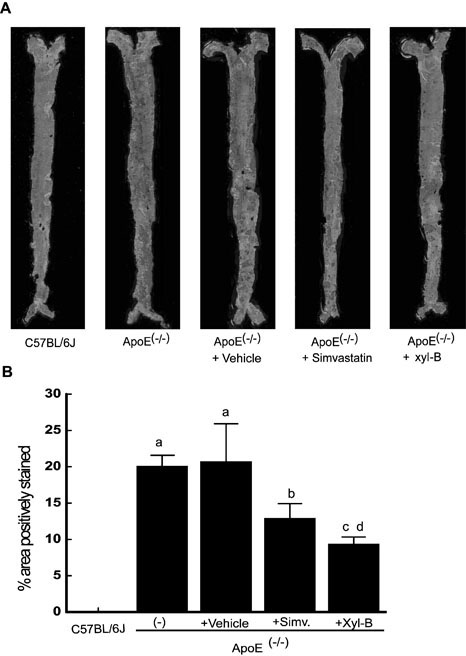
[0018] Example 1 Effect of Xyloketal B on Plaque Area in Atherosclerotic Mice
[0019] (1) Materials and methods
[0020] 1. Drugs:
[0021] Xyloketal B, white powder. It was dissolved in DMSO first, the storage concentration was 80mmol / L, and it was stored at 4°C for later use. It was diluted with 10% propylene glycol saline, and administered by intraperitoneal injection (the final concentration of Xyloketal B was 14mg / kg·d). Simvastatin was purchased from Hangzhou Merck Pharmaceutical Co., Ltd. (40 mg tablet, batch number 07279), and it was ground into powder during administration, dissolved in deionized water and administered intragastrically (the final concentration of simvastatin was 10 mg / kg·d) .
[0022] 2. Grouping, feeding and administration of experimental animals
[0023] In this experiment, apoE knockout mice (strain C57BL / 6J, male, clean grade, 5 weeks old) and wild-type male C57BL / 6J mice were selected. All animals were adaptively fed for 1 week, and wer...
Embodiment 2
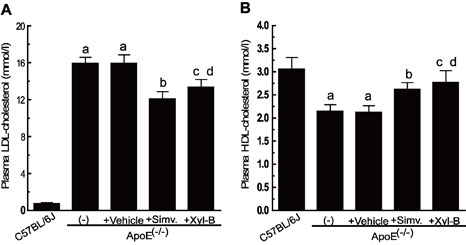
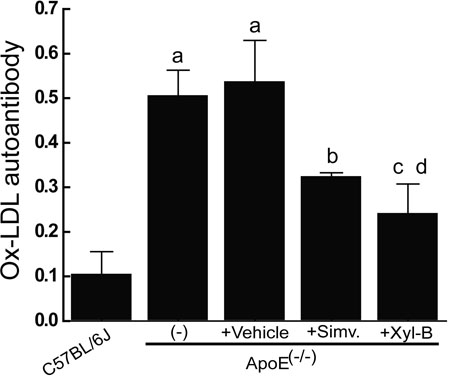
[0045] Example 2 Effect of Xyloketal B on Blood Lipid Level and Anti-Ox-LDL Antibody in Atherosclerotic Mice
[0046] (1) Materials and methods
[0047] 1. Drug preparation, animal model grouping, administration see Example 1
[0048] 2. Plasma and reagents Fresh plasma from healthy people was purchased from the Department of Hematology, The First Affiliated Hospital of Sun Yat-sen University, Ethylene glycol-bis(2-aminoethylether) -N,N,N', N'-tetraacetic acid (EGTA), polyethylene glycol (PEG 6000), agarose (agarose), and Coomassie Brilliant Blue G-250 were all purchased from Sigma. Enzymatic detection of blood lipid analysis direct high-density lipoprotein cholesterol (HDL-C) kit, direct low-density lipoprotein cholesterol (LDL-C) kit. Purchased from Zhongsheng Beikong Biotechnology Co., Ltd. Serum anti-Ox-LDL antibody assay kit (Protein Detector TM ELISA Kit) was purchased from Kirkegaard Perry Labs.
[0049] 3. Separation, purification and oxidation of low density...
Embodiment 3
[0069] Example 3 Effects of Xyloketal B on plaque components and arterial endothelial injury in atherosclerotic mice
[0070] (1) Materials and methods
[0071] 1. See Example 1 for drug preparation, animal model grouping, and administration methods
[0072] 2. Other experimental materials: Mac-3 (BD Biosciences), CD31 ([PECAM-1]) (BD Pharmingen). Anti-shedding glass slide, microscope cover glass, mouse two-step detection kit, rabbit two-step detection kit, DAB chromogenic kit, immunofluorescent secondary antibody (all purchased from Beijing Zhongshan Jinqiao Biological Company), neutral Resin glue (Beijing Chemical Reagent No. 4 Factory), acetone (Guangzhou Chemical Reagent Co., Ltd.).
[0073] 3. Immunohistochemistry and immunofluorescence detection methods
[0074] Immunohistochemical experimental steps: (1) Frozen sections were fixed with acetone at -20°C for 10 minutes and washed with PBS for 5 minutes. (2) 0.3% H 2 o 2 Methanol solution (to block endogenou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com