Macrocyclic aromatic-amine structured compound as well as preparation method and application thereof
A compound, ring-type technology, applied in the synthesis and application of hole transport materials, can solve the problems of destroying the uniformity and isotropy of the film, failing to disclose superior performance, and reducing device efficiency, and achieve stable color purity and photoelectricity. Efficiency, maximum fluorescence brightness improvement, easy hole injection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
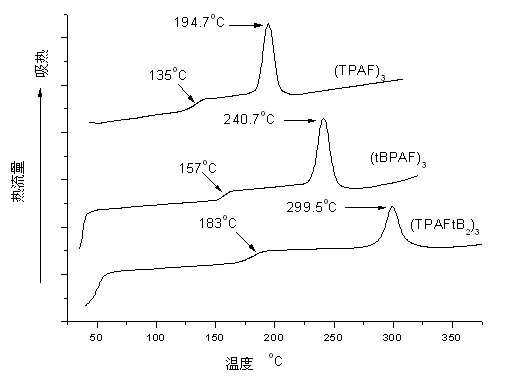
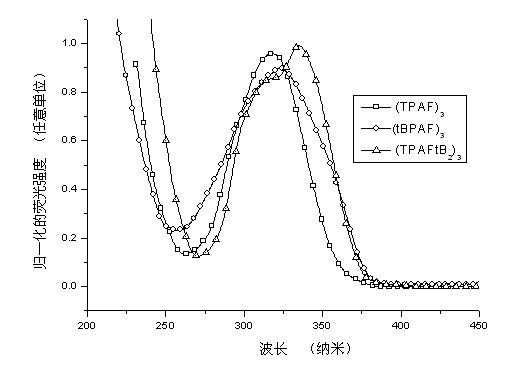
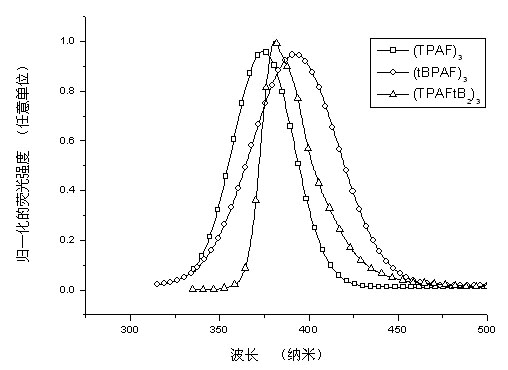
Image
Examples
Embodiment 1
[0106] compound a 4 Synthesis
[0107] In a 250mL single-necked flask, add 9mmol of magnesium chips and a piece of iodine, under the protection of an inert gas, stir and heat the magnesium chips, while slowly adding 10 mmol of 4-bromo-N-phenyl-N-p-methyl 50mL tetrahydrofuran solution of aniline, until the reaction of magnesium flakes is completed, slowly add 50mL tetrahydrofuran solution dissolved in 10mmol fluorenone dropwise, reflux for two hours after dropping, drop to room temperature, add dropwise 2M ammonium chloride solution to adjust the pH value to 7. The organic phase was separated, the inorganic phase was extracted three times with anhydrous ether, and combined with the organic phase. The combined organic phases were dried with an appropriate amount of anhydrous magnesium sulfate, the solvent was removed, and the chromatographic column was separated to obtain a yellow solid compound a 4 . The conversion rate was 71%.
[0108] 1 H NMR (500MHz, CDCl 3 , δ(ppm)):...
Embodiment 2
[0110] (TPAF) 3 Synthesis of macrocycles
[0111] In a 100mL one-necked flask, add compound a 40.6 g, add 80 mL of 1,3,5-mesitylene, drop 2 drops of methanesulfonic acid, then add a condenser, and react at 160°C for 20 hours under the protection of nitrogen. Cool down, remove the solvent, and separate and purify through a silica gel column to obtain a light yellow solid with a yield of 78%.
[0112] 1 H NMR (500MHz, CDCl 3 , δ (ppm)): 2.30 (s, 9H); 6.84 (d, J = 8.5Hz, 6H); 6.88 (d, J = 8.0Hz, 6H); 6.98 (d, J = 7.0Hz, 12H); 7.13~7.15(m, 12H); 7.31(t, J=8.0Hz, 6H); 7.37(t, J=8.0Hz, 6H); 7.49(d, J=8.0Hz, 6H); 7.77(t, J =7.0Hz, 6H). MS (MALDI-TOF) m / z: 1264.99 [M+H] + . Anal. Calcd. for (TPAF) 3 (C 96 h 69 N 3 ): C, 91.18; H, 5.50; N, 3.32. Found: C, 90.53; H, 5.89; N, 3.03.
Embodiment 3
[0114] compound a 5 Synthesis
[0115] In a 100mL single-necked flask, add 10mmol of N-(4-(p-tert-butylphenyl)-phenyl)-N-(4'-bromophenyl)aniline, add 50mL of dried tetrahydrofuran under nitrogen protection, and cool down To -78°C, slowly add 4.2mL of 2.5M butyllithium solution dropwise, and after 1 hour, slowly add dropwise 30mL tetrahydrofuran dissolved in 9.5mmol fluorenone, after the drop, keep the temperature at -78°C for 1 hour, and then spontaneously rise to Ambient temperature; slowly add 2M hydrochloric acid dropwise to bring the pH value to 7, after cooling, separate the organic phase, extract the inorganic phase with anhydrous ether three times, and combine with the organic phase. The combined organic phases were dried with anhydrous magnesium sulfate, the solvent was removed, and the chromatographic column was separated to obtain a yellow solid compound a 5 . The conversion rate was 69%.
[0116] 1 H NMR (500MHz, CDCl 3 , δ(ppm)): 1.35(s, 9H); 6.93(t, J=8.0Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com